Fish Models for Exploring Mitochondrial Dysfunction Affecting Neurodegenerative Disorders
- PMID: 37108237
- PMCID: PMC10138900
- DOI: 10.3390/ijms24087079
Fish Models for Exploring Mitochondrial Dysfunction Affecting Neurodegenerative Disorders
Abstract
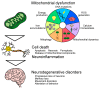
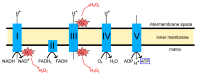
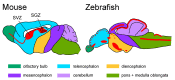
Neurodegenerative disorders are characterized by the progressive loss of neuronal structure or function, resulting in memory loss and movement disorders. Although the detailed pathogenic mechanism has not been elucidated, it is thought to be related to the loss of mitochondrial function in the process of aging. Animal models that mimic the pathology of a disease are essential for understanding human diseases. In recent years, small fish have become ideal vertebrate models for human disease due to their high genetic and histological homology to humans, ease of in vivo imaging, and ease of genetic manipulation. In this review, we first outline the impact of mitochondrial dysfunction on the progression of neurodegenerative diseases. Then, we highlight the advantages of small fish as model organisms, and present examples of previous studies regarding mitochondria-related neuronal disorders. Lastly, we discuss the applicability of the turquoise killifish, a unique model for aging research, as a model for neurodegenerative diseases. Small fish models are expected to advance our understanding of the mitochondrial function in vivo, the pathogenesis of neurodegenerative diseases, and be important tools for developing therapies to treat diseases.
Keywords: medaka; mitochondria; neurodegenerative disorders; turquoise killifish; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
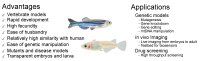
Similar articles
-
Zebrafish, Medaka and Turquoise Killifish for Understanding Human Neurodegenerative/Neurodevelopmental Disorders.Int J Mol Sci. 2022 Jan 26;23(3):1399. doi: 10.3390/ijms23031399. Int J Mol Sci. 2022. PMID: 35163337 Free PMC article. Review.
-
A review on neurodegeneration in the fast-ageing killifish, the first animal model to study the natural occurrence of neuronal cell loss.Ageing Res Rev. 2023 Nov;91:102065. doi: 10.1016/j.arr.2023.102065. Epub 2023 Sep 3. Ageing Res Rev. 2023. PMID: 37666433 Review.
-
The African turquoise killifish: A research organism to study vertebrate aging and diapause.Aging Cell. 2018 Jun;17(3):e12757. doi: 10.1111/acel.12757. Epub 2018 Mar 24. Aging Cell. 2018. PMID: 29573324 Free PMC article. Review.
-
Turquoise killifish: A natural model of age-dependent brain degeneration.Ageing Res Rev. 2023 Sep;90:102019. doi: 10.1016/j.arr.2023.102019. Epub 2023 Jul 22. Ageing Res Rev. 2023. PMID: 37482345 Review.
-
The short-lived African turquoise killifish: an emerging experimental model for ageing.Dis Model Mech. 2016 Feb;9(2):115-29. doi: 10.1242/dmm.023226. Dis Model Mech. 2016. PMID: 26839399 Free PMC article. Review.
Cited by
-
Evaluation of glycyrrhetinic acid in attenuating adverse effects of a high-fat diet in largemouth bass (Micropterus salmoides).Anim Nutr. 2024 Sep 23;19:248-260. doi: 10.1016/j.aninu.2024.09.002. eCollection 2024 Dec. Anim Nutr. 2024. PMID: 39640558 Free PMC article.
-
Preclinical models of mitochondrial dysfunction: mtDNA and nuclear-encoded regulators in diverse pathologies.Front Aging. 2025 Jun 23;6:1585508. doi: 10.3389/fragi.2025.1585508. eCollection 2025. Front Aging. 2025. PMID: 40625678 Free PMC article. Review.
-
Captivating Colors, Crucial Roles: Astaxanthin's Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance.Animals (Basel). 2023 Oct 29;13(21):3357. doi: 10.3390/ani13213357. Animals (Basel). 2023. PMID: 37958112 Free PMC article. Review.
-
Homozygous slc25a20 zebrafish mutant reveals insights into carnitine-acylcarnitine translocase deficiency pathogenesis.Mol Genet Metab Rep. 2024 Nov 22;41:101165. doi: 10.1016/j.ymgmr.2024.101165. eCollection 2024 Dec. Mol Genet Metab Rep. 2024. PMID: 39650084 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

