Ca2+ Dependent Formation/Collapse of Cylindrical Ca2+-ATPase Crystals in Scallop Sarcoplasmic Reticulum (SR) Vesicles: A Possible Dynamic Role of SR in Regulation of Muscle Contraction
- PMID: 37108240
- PMCID: PMC10139091
- DOI: 10.3390/ijms24087080
Ca2+ Dependent Formation/Collapse of Cylindrical Ca2+-ATPase Crystals in Scallop Sarcoplasmic Reticulum (SR) Vesicles: A Possible Dynamic Role of SR in Regulation of Muscle Contraction
Abstract
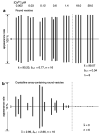
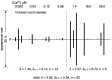
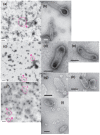
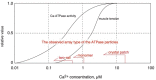
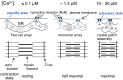
[Ca2+]-dependent crystallization of the Ca2+-ATPase molecules in sarcoplasmic reticulum (SR) vesicles isolated from scallop striated muscle elongated the vesicles in the absence of ATP, and ATP stabilized the crystals. Here, to determine the [Ca2+]-dependence of vesicle elongation in the presence of ATP, SR vesicles in various [Ca2+] environments were imaged using negative stain electron microscopy. The images obtained revealed the following phenomena. (i) Crystal-containing elongated vesicles appeared at ≤1.4 µM Ca2+ and almost disappeared at ≥18 µM Ca2+, where ATPase activity reaches its maximum. (ii) At ≥18 µM Ca2+, almost all SR vesicles were in the round form and covered by tightly clustered ATPase crystal patches. (iii) Round vesicles dried on electron microscopy grids occasionally had cracks, probably because surface tension crushed the solid three-dimensional spheres. (iv) [Ca2+]-dependent ATPase crystallization was rapid (<1 min) and reversible. These data prompt the hypothesis that SR vesicles autonomously elongate or contract with the help of a calcium-sensitive ATPase network/endoskeleton and that ATPase crystallization may modulate physical properties of the SR architecture, including the ryanodine receptors that control muscle contraction.
Keywords: Ca2+-ATPase; SR contraction; SR elongation; calcium; cell dynamics; cell morphology; ryanodine receptor; sarcoplasmic reticulum (SR); scallop; transmission microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Elongation and Contraction of Scallop Sarcoplasmic Reticulum (SR): ATP Stabilizes Ca2+-ATPase Crystalline Array Elongation of SR Vesicles.Int J Mol Sci. 2022 Mar 18;23(6):3311. doi: 10.3390/ijms23063311. Int J Mol Sci. 2022. PMID: 35328731 Free PMC article.
-
Ca2+-ATPase Molecules as a Calcium-Sensitive Membrane-Endoskeleton of Sarcoplasmic Reticulum.Int J Mol Sci. 2021 Mar 5;22(5):2624. doi: 10.3390/ijms22052624. Int J Mol Sci. 2021. PMID: 33807779 Free PMC article.
-
Comparison of the effects of fluoride on the calcium pumps of cardiac and fast skeletal muscle sarcoplasmic reticulum: evidence for tissue-specific qualitative difference in calcium-induced pump conformation.Biochim Biophys Acta. 1994 May 11;1191(2):231-43. doi: 10.1016/0005-2736(94)90174-0. Biochim Biophys Acta. 1994. PMID: 8172909
-
Ca(2+ )transport by the sarcoplasmic reticulum Ca(2+)-ATPase in sea cucumber (Ludwigothurea grisea) muscle.J Exp Biol. 2001 Mar;204(Pt 5):909-21. doi: 10.1242/jeb.204.5.909. J Exp Biol. 2001. PMID: 11171414 Review.
-
A comparative study of the Ca2+-Mg2+ dependent ATPase from skeletal muscles of young, adult and old rats.Mech Ageing Dev. 1989 Aug;49(2):105-117. doi: 10.1016/0047-6374(89)90094-8. Mech Ageing Dev. 1989. PMID: 2529400 Review.
References
-
- Sanger J.W., Sanger J.M. Sarcoplasmic reticulum in the adductor muscle of a Bermuda scallop: Comparison of smooth versus cross-striated portions. Biol. Bull. 1985;168:447–460. doi: 10.2307/1541524. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

