Adhesion to the Brain Endothelium Selects Breast Cancer Cells with Brain Metastasis Potential
- PMID: 37108248
- PMCID: PMC10138870
- DOI: 10.3390/ijms24087087
Adhesion to the Brain Endothelium Selects Breast Cancer Cells with Brain Metastasis Potential
Abstract
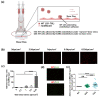
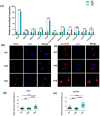
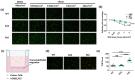
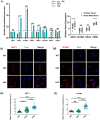
Tumor cells metastasize from a primary lesion to distant organs mainly through hematogenous dissemination, in which tumor cell re-adhesion to the endothelium is essential before extravasating into the target site. We thus hypothesize that tumor cells with the ability to adhere to the endothelium of a specific organ exhibit enhanced metastatic tropism to this target organ. This study tested this hypothesis and developed an in vitro model to mimic the adhesion between tumor cells and brain endothelium under fluid shear stress, which selected a subpopulation of tumor cells with enhanced adhesion strength. The selected cells up-regulated the genes related to brain metastasis and exhibited an enhanced ability to transmigrate through the blood-brain barrier. In the soft microenvironments that mimicked brain tissue, these cells had elevated adhesion and survival ability. Further, tumor cells selected by brain endothelium adhesion expressed higher levels of MUC1, VCAM1, and VLA-4, which were relevant to breast cancer brain metastasis. In summary, this study provides the first piece of evidence to support that the adhesion of circulating tumor cells to the brain endothelium selects the cells with enhanced brain metastasis potential.
Keywords: biomechanics; brain metastasis; endothelial adhesion; fluid shear stress; mechanobiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The effect of soluble E-selectin on tumor progression and metastasis.BMC Cancer. 2016 May 24;16:331. doi: 10.1186/s12885-016-2366-2. BMC Cancer. 2016. PMID: 27220365 Free PMC article.
-
Hemodynamic shear flow regulates biophysical characteristics and functions of circulating breast tumor cells reminiscent of brain metastasis.Soft Matter. 2018 Dec 5;14(47):9528-9533. doi: 10.1039/c8sm01781f. Soft Matter. 2018. PMID: 30468439
-
Adhesion molecules and their role in cancer metastasis.Cell Biophys. 1993 Aug-Dec;23(1-3):3-89. doi: 10.1007/BF02796507. Cell Biophys. 1993. PMID: 7895250 Review.
-
Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium.Clin Cancer Res. 2011 Nov 15;17(22):7035-46. doi: 10.1158/1078-0432.CCR-11-1462. Epub 2011 Sep 20. Clin Cancer Res. 2011. PMID: 21933892
-
Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both?Cell Adh Migr. 2015;9(5):345-56. doi: 10.1080/19336918.2015.1059563. Epub 2015 Aug 27. Cell Adh Migr. 2015. PMID: 26312653 Free PMC article. Review.
Cited by
-
Breast Tumor Metastasis and Its Microenvironment: It Takes Both Seed and Soil to Grow a Tumor and Target It for Treatment.Cancers (Basel). 2024 Feb 23;16(5):911. doi: 10.3390/cancers16050911. Cancers (Basel). 2024. PMID: 38473273 Free PMC article. Review.
-
Effects of fluid shear stress duration on the mechanical properties of HeLa cells using atomic force microscopy.PLoS One. 2025 May 5;20(5):e0321296. doi: 10.1371/journal.pone.0321296. eCollection 2025. PLoS One. 2025. PMID: 40323916 Free PMC article.
-
Invasion and metastasis in cancer: molecular insights and therapeutic targets.Signal Transduct Target Ther. 2025 Feb 21;10(1):57. doi: 10.1038/s41392-025-02148-4. Signal Transduct Target Ther. 2025. PMID: 39979279 Free PMC article. Review.
-
Blood-Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models.Int J Mol Sci. 2023 Aug 11;24(16):12699. doi: 10.3390/ijms241612699. Int J Mol Sci. 2023. PMID: 37628879 Free PMC article. Review.
-
Metastatic brain tumors: from development to cutting-edge treatment.MedComm (2020). 2024 Dec 20;6(1):e70020. doi: 10.1002/mco2.70020. eCollection 2025 Jan. MedComm (2020). 2024. PMID: 39712454 Free PMC article. Review.
References
-
- Follain G., Osmani N., Azevedo A.S., Allio G., Mercier L., Karreman M.A., Solecki G., Leòn M.J.G., Lefebvre O., Fekonja N., et al. Hemodynamic Forces Tune the Arrest, Adhesion, and Extravasation of Circulating Tumor Cells. Dev. Cell. 2018;45:33–52.e12. doi: 10.1016/j.devcel.2018.02.015. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

