The Blinin Accumulation Promoted by CbMYB32 Involved in Conyza blinii Resistance to Nocturnal Low Temperature
- PMID: 37108302
- PMCID: PMC10139108
- DOI: 10.3390/ijms24087143
The Blinin Accumulation Promoted by CbMYB32 Involved in Conyza blinii Resistance to Nocturnal Low Temperature
Abstract
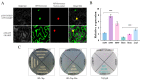
Blinin, a unique terpenoid from Conyza blinii (C. blinii), benefits our health even though this is not its primary function. Physiological and ecological studies have found that the great secondary metabolites participate in important biological processes and relate to species evolution, environmental adaptation, and so on. Moreover, our previous studies have shown that the metabolism and accumulation of blinin has a close correspondence with nocturnal low temperature (NLT). To find out the transcriptional regulation linker in the crosstalk between blinin and NLT, RNA-seq, comparative analysis, and co-expression network were performed. The results indicated that CbMYB32 is located in a nucleus without independent transcriptional activation activity and is probably involved in the metabolism of blinin. Furthermore, we compared the silence and overexpression of CbMYB32 with wild C. blinii. Compared with the overexpression and the wildtype, the CbMYB32 silence line lost more than half of the blinin and detected more peroxide under NLT. Finally, as a characteristic secret of C. blinii, it is reasonable to infer that blinin participates in the NLT adaptation mechanism and has contributed to the systematic evolution of C. blinii.
Keywords: Conyza blinii; nocturnal low temperature resistance; transcriptional regulation; unique terpenoid blinin; virus-induced gene silencing.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




Similar articles
-
Enhanced antioxidant capacity and upregulated transporter genes contribute to the UV-B-induced increase in blinin in Conyza blinii.Environ Sci Pollut Res Int. 2021 Mar;28(11):13275-13287. doi: 10.1007/s11356-020-11502-8. Epub 2020 Nov 11. Environ Sci Pollut Res Int. 2021. PMID: 33175358
-
Ferrous iron-induced increases in capitate glandular trichome density and upregulation of CbHO-1 contributes to increases in blinin content in Conyza blinii.Planta. 2020 Oct 10;252(5):81. doi: 10.1007/s00425-020-03492-1. Planta. 2020. PMID: 33037484
-
ABA and SA Participate in the Regulation of Terpenoid Metabolic Flux Induced by Low-Temperature within Conyza blinii.Life (Basel). 2023 Jan 29;13(2):371. doi: 10.3390/life13020371. Life (Basel). 2023. PMID: 36836728 Free PMC article.
-
The jasmonate-responsive transcription factor CbWRKY24 regulates terpenoid biosynthetic genes to promote saponin biosynthesis in Conyza blinii H. Lév.J Genet. 2018 Dec;97(5):1379-1388. J Genet. 2018. PMID: 30555086
-
Biology and management of two important Conyza weeds: a global review.Environ Sci Pollut Res Int. 2016 Dec;23(24):24694-24710. doi: 10.1007/s11356-016-7794-7. Epub 2016 Oct 31. Environ Sci Pollut Res Int. 2016. PMID: 27798798 Review.
Cited by
-
RNAi technology development for weed control: all smoke and no fire?Pest Manag Sci. 2025 Jul;81(7):3430-3436. doi: 10.1002/ps.8729. Epub 2025 Feb 21. Pest Manag Sci. 2025. PMID: 39980431 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

