Aberrant Dopamine System Function in the Ferrous Amyloid Buthionine (FAB) Rat Model of Alzheimer's Disease
- PMID: 37108357
- PMCID: PMC10138591
- DOI: 10.3390/ijms24087196
Aberrant Dopamine System Function in the Ferrous Amyloid Buthionine (FAB) Rat Model of Alzheimer's Disease
Abstract
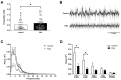
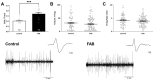
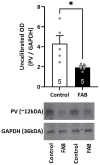
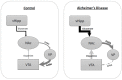
Antipsychotics increase the risk of death in elderly patients with Alzheimer's disease (AD). Thus, there is an immediate need for novel therapies to treat comorbid psychosis in AD. Psychosis has been attributed to a dysregulation of the dopamine system and is associated with aberrant regulation by the hippocampus. Given that the hippocampus is a key site of pathology in AD, we posit that aberrant regulation of the dopamine system may contribute to comorbid psychosis in AD. A ferrous amyloid buthionine (FAB) rodent model was used to model a sporadic form of AD. FAB rats displayed functional hippocampal alterations, which were accompanied by decreases in spontaneous, low-frequency oscillations and increases in the firing rates of putative pyramidal neurons. Additionally, FAB rats exhibited increases in dopamine neuron population activity and augmented responses to the locomotor-inducing effects of MK-801, as is consistent with rodent models of psychosis-like symptomatology. Further, working memory deficits in the Y-maze, consistent with an AD-like phenotype, were observed in FAB rats. These data suggest that the aberrant hippocampal activity observed in AD may contribute to dopamine-dependent psychosis, and that the FAB model may be useful for the investigation of comorbid psychosis related to AD. Understanding the pathophysiology that leads to comorbid psychosis in AD will ultimately lead to the discovery of novel targets for the treatment of this disease.
Keywords: Alzheimer’s disease; FAB; dopamine; in vivo electrophysiology; psychosis; ventral hippocampus.
Conflict of interest statement
Lodge has received research funding from Heptares Therapeutics Ltd. and consulting fees from Alkermes, Inc., and is co-inventor of a patent (US 2019/0117637). All of these are unrelated to the work described in this manuscript.
Figures

Similar articles
-
α5-GABAA Receptor Modulation Reverses Behavioral and Neurophysiological Correlates of Psychosis in Rats with Ventral Hippocampal Alzheimer's Disease-like Pathology.Int J Mol Sci. 2023 Jul 22;24(14):11788. doi: 10.3390/ijms241411788. Int J Mol Sci. 2023. PMID: 37511546 Free PMC article.
-
The effects of astaxanthin treatment on a rat model of Alzheimer's disease.Brain Res Bull. 2021 Jul;172:151-163. doi: 10.1016/j.brainresbull.2021.04.020. Epub 2021 Apr 28. Brain Res Bull. 2021. PMID: 33932491
-
The synergy of β amyloid 1-42 and oxidative stress in the development of Alzheimer's disease-like neurodegeneration of hippocampal cells.Sci Rep. 2022 Oct 25;12(1):17883. doi: 10.1038/s41598-022-22761-5. Sci Rep. 2022. PMID: 36284177 Free PMC article.
-
Adolescent stress contributes to aberrant dopamine signaling in a heritable rodent model of susceptibility.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Dec 20;95:109701. doi: 10.1016/j.pnpbp.2019.109701. Epub 2019 Jul 10. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 31299274 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
-
α5-GABAA Receptor Modulation Reverses Behavioral and Neurophysiological Correlates of Psychosis in Rats with Ventral Hippocampal Alzheimer's Disease-like Pathology.Int J Mol Sci. 2023 Jul 22;24(14):11788. doi: 10.3390/ijms241411788. Int J Mol Sci. 2023. PMID: 37511546 Free PMC article.
-
An Expanded Narrative Review of Neurotransmitters on Alzheimer's Disease: The Role of Therapeutic Interventions on Neurotransmission.Mol Neurobiol. 2025 Feb;62(2):1631-1674. doi: 10.1007/s12035-024-04333-y. Epub 2024 Jul 16. Mol Neurobiol. 2025. PMID: 39012443 Free PMC article. Review.
References
-
- Emanuel J.E., Lopez O.L., Houck P.R., Becker J.T., Weamer E.A., Demichele-Sweet M.A., Kuller L., Sweet R.A. Trajectory of cognitive decline as a predictor of psychosis in early Alzheimer disease in the cardiovascular health study. Am. J. Geriatr. Psychiatry. 2011;19:160–168. doi: 10.1097/JGP.0b013e3181e446c8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

