NLRC5-CIITA Fusion Protein as an Effective Inducer of MHC-I Expression and Antitumor Immunity
- PMID: 37108368
- PMCID: PMC10138588
- DOI: 10.3390/ijms24087206
NLRC5-CIITA Fusion Protein as an Effective Inducer of MHC-I Expression and Antitumor Immunity
Abstract
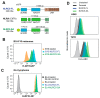
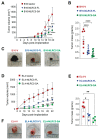
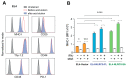
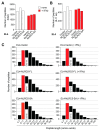
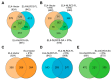
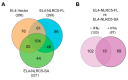
Aggressive tumors evade cytotoxic T lymphocytes by suppressing MHC class-I (MHC-I) expression that also compromises tumor responsiveness to immunotherapy. MHC-I defects strongly correlate to defective expression of NLRC5, the transcriptional activator of MHC-I and antigen processing genes. In poorly immunogenic B16 melanoma cells, restoring NLRC5 expression induces MHC-I and elicits antitumor immunity, raising the possibility of using NLRC5 for tumor immunotherapy. As the clinical application of NLRC5 is constrained by its large size, we examined whether a smaller NLRC5-CIITA fusion protein, dubbed NLRC5-superactivator (NLRC5-SA) as it retains the ability to induce MHC-I, could be used for tumor growth control. We show that stable NLRC5-SA expression in mouse and human cancer cells upregulates MHC-I expression. B16 melanoma and EL4 lymphoma tumors expressing NLRC5-SA are controlled as efficiently as those expressing full-length NLRC5 (NLRC5-FL). Comparison of MHC-I-associated peptides (MAPs) eluted from EL4 cells expressing NLRC5-FL or NLRC5-SA and analyzed by mass spectrometry revealed that both NLRC5 constructs expanded the MAP repertoire, which showed considerable overlap but also included a substantial proportion of distinct peptides. Thus, we propose that NLRC5-SA, with its ability to increase tumor immunogenicity and promote tumor growth control, could overcome the limitations of NLRC5-FL for translational immunotherapy applications.
Keywords: B16-F10; EL4; MHC-I; MHC-I associated peptides; NLRC5; NLRC5-SA; tumor immunogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The N-terminal domain of NLRC5 confers transcriptional activity for MHC class I and II gene expression.J Immunol. 2014 Sep 15;193(6):3090-100. doi: 10.4049/jimmunol.1401065. Epub 2014 Aug 15. J Immunol. 2014. PMID: 25127861
-
NLRC5 elicits antitumor immunity by enhancing processing and presentation of tumor antigens to CD8(+) T lymphocytes.Oncoimmunology. 2016 Mar 28;5(6):e1151593. doi: 10.1080/2162402X.2016.1151593. eCollection 2016 Jun. Oncoimmunology. 2016. PMID: 27471621 Free PMC article.
-
The MHC Class-I Transactivator NLRC5: Implications to Cancer Immunology and Potential Applications to Cancer Immunotherapy.Int J Mol Sci. 2021 Feb 17;22(4):1964. doi: 10.3390/ijms22041964. Int J Mol Sci. 2021. PMID: 33671123 Free PMC article. Review.
-
NLRC5: a newly discovered MHC class I transactivator (CITA).Microbes Infect. 2012 Jun;14(6):477-84. doi: 10.1016/j.micinf.2011.12.007. Epub 2011 Dec 22. Microbes Infect. 2012. PMID: 22209772 Free PMC article. Review.
-
NLRC5 exclusively transactivates MHC class I and related genes through a distinctive SXY module.PLoS Genet. 2015 Mar 26;11(3):e1005088. doi: 10.1371/journal.pgen.1005088. eCollection 2015 Mar. PLoS Genet. 2015. PMID: 25811463 Free PMC article.
Cited by
-
Development and validation of optimized lentivirus-like particles for gene editing tool delivery with Gag-Only strategy.Eur J Med Res. 2025 Apr 4;30(1):242. doi: 10.1186/s40001-025-02499-2. Eur J Med Res. 2025. PMID: 40186294 Free PMC article.
-
Response to "NLRC5 germline variants and their potential role in eliciting an immune response in patients with cancer treated with immune checkpoint inhibitors" by Xiang-Yu Meng.J Immunother Cancer. 2023 Jun;11(6):e007397. doi: 10.1136/jitc-2023-007397. J Immunother Cancer. 2023. PMID: 37349129 Free PMC article. No abstract available.
-
NLRC5 overexpression in ovarian tumors remodels the tumor microenvironment and increases T-cell reactivity toward autologous tumor-associated antigens.Front Immunol. 2024 Jan 3;14:1295208. doi: 10.3389/fimmu.2023.1295208. eCollection 2023. Front Immunol. 2024. PMID: 38235131 Free PMC article.
-
NLRC5 exerts anti-endometriosis effects through inhibiting ERβ-mediated inflammatory response.BMC Med. 2024 Sep 2;22(1):351. doi: 10.1186/s12916-024-03571-0. BMC Med. 2024. PMID: 39218863 Free PMC article.
-
New insights into the structure domain and function of NLR family CARD domain containing 5.Cell Commun Signal. 2025 Jan 23;23(1):42. doi: 10.1186/s12964-024-02012-y. Cell Commun Signal. 2025. PMID: 39849460 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

