Extracellular Vesicles in Breast Cancer: From Biology and Function to Clinical Diagnosis and Therapeutic Management
- PMID: 37108371
- PMCID: PMC10139222
- DOI: 10.3390/ijms24087208
Extracellular Vesicles in Breast Cancer: From Biology and Function to Clinical Diagnosis and Therapeutic Management
Abstract
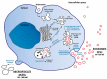
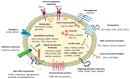
Breast cancer (BC) is the first worldwide most frequent cancer in both sexes and the most commonly diagnosed in females. Although BC mortality has been thoroughly declining over the past decades, there are still considerable differences between women diagnosed with early BC and when metastatic BC is diagnosed. BC treatment choice is widely dependent on precise histological and molecular characterization. However, recurrence or distant metastasis still occurs even with the most recent efficient therapies. Thus, a better understanding of the different factors underlying tumor escape is mainly mandatory. Among the leading candidates is the continuous interplay between tumor cells and their microenvironment, where extracellular vesicles play a significant role. Among extracellular vesicles, smaller ones, also called exosomes, can carry biomolecules, such as lipids, proteins, and nucleic acids, and generate signal transmission through an intercellular transfer of their content. This mechanism allows tumor cells to recruit and modify the adjacent and systemic microenvironment to support further invasion and dissemination. By reciprocity, stromal cells can also use exosomes to profoundly modify tumor cell behavior. This review intends to cover the most recent literature on the role of extracellular vesicle production in normal and cancerous breast tissues. Specific attention is paid to the use of extracellular vesicles for early BC diagnosis, follow-up, and prognosis because exosomes are actually under the spotlight of researchers as a high-potential source of liquid biopsies. Extracellular vesicles in BC treatment as new targets for therapy or efficient nanovectors to drive drug delivery are also summarized.
Keywords: breast cancer; diagnostic; exosomes; extracellular vesicles; prognosis; targeting; therapy; vector.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gennari A., André F., Barrios C.H., Cortés J., de Azambuja E., DeMichele A., Dent R., Fenlon D., Gligorov J., Hurvitz S.A., et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

