Investigation of the Compatibility between Warheads and Peptidomimetic Sequences of Protease Inhibitors-A Comprehensive Reactivity and Selectivity Study
- PMID: 37108388
- PMCID: PMC10138721
- DOI: 10.3390/ijms24087226
Investigation of the Compatibility between Warheads and Peptidomimetic Sequences of Protease Inhibitors-A Comprehensive Reactivity and Selectivity Study
Abstract
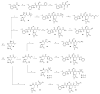
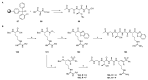
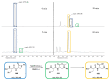
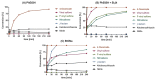
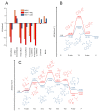
Covalent peptidomimetic protease inhibitors have gained a lot of attention in drug development in recent years. They are designed to covalently bind the catalytically active amino acids through electrophilic groups called warheads. Covalent inhibition has an advantage in terms of pharmacodynamic properties but can also bear toxicity risks due to non-selective off-target protein binding. Therefore, the right combination of a reactive warhead with a well-suited peptidomimetic sequence is of great importance. Herein, the selectivities of well-known warheads combined with peptidomimetic sequences suited for five different proteases were investigated, highlighting the impact of both structure parts (warhead and peptidomimetic sequence) for affinity and selectivity. Molecular docking gave insights into the predicted binding modes of the inhibitors inside the binding pockets of the different enzymes. Moreover, the warheads were investigated by NMR and LC-MS reactivity assays against serine/threonine and cysteine nucleophile models, as well as by quantum mechanics simulations.
Keywords: covalent inhibitors; in vitro study; peptidomimetic sequence; protease inhibitors; reactivity and selectivity study; warhead.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



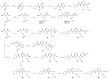







Similar articles
-
Characterising covalent warhead reactivity.Bioorg Med Chem. 2019 May 15;27(10):2066-2074. doi: 10.1016/j.bmc.2019.04.002. Epub 2019 Apr 3. Bioorg Med Chem. 2019. PMID: 30975501 Free PMC article.
-
Electrophilic warheads in covalent drug discovery: an overview.Expert Opin Drug Discov. 2022 Apr;17(4):413-422. doi: 10.1080/17460441.2022.2034783. Epub 2022 Feb 6. Expert Opin Drug Discov. 2022. PMID: 35129005 Review.
-
A new method for filtering of reactive "warheads" of transition-state analog protease inhibitors.Eur J Med Chem. 2014 Apr 22;77:134-8. doi: 10.1016/j.ejmech.2014.02.059. Epub 2014 Feb 28. Eur J Med Chem. 2014. PMID: 24631732
-
Covalent Reversible Inhibitors of Cysteine Proteases Containing the Nitrile Warhead: Recent Advancement in the Field of Viral and Parasitic Diseases.Molecules. 2022 Apr 15;27(8):2561. doi: 10.3390/molecules27082561. Molecules. 2022. PMID: 35458759 Free PMC article. Review.
-
Peptidomimetic nitrile warheads as SARS-CoV-2 3CL protease inhibitors.RSC Med Chem. 2021 Aug 20;12(10):1722-1730. doi: 10.1039/d1md00247c. eCollection 2021 Oct 20. RSC Med Chem. 2021. PMID: 34778773 Free PMC article.
Cited by
-
Neutral pH-Selective Inhibition of Cytosolic Cathepsin B: A Novel Drug Targeting Strategy for Traumatic Brain Injury and Alzheimer's Disease.ACS Chem Biol. 2025 Aug 15;20(8):1841-1848. doi: 10.1021/acschembio.5c00463. Epub 2025 Jul 23. ACS Chem Biol. 2025. PMID: 40700403 Free PMC article. Review.
-
Peptidyl nitroalkene inhibitors of main protease rationalized by computational and crystallographic investigations as antivirals against SARS-CoV-2.Commun Chem. 2024 Jan 18;7(1):15. doi: 10.1038/s42004-024-01104-7. Commun Chem. 2024. PMID: 38238420 Free PMC article.
-
Recent Advances on Targeting Proteases for Antiviral Development.Viruses. 2024 Feb 27;16(3):366. doi: 10.3390/v16030366. Viruses. 2024. PMID: 38543732 Free PMC article. Review.
-
Ligand-Based Design of Selective Peptidomimetic uPA and TMPRSS2 Inhibitors with Arg Bioisosteres.Int J Mol Sci. 2024 Jan 23;25(3):1375. doi: 10.3390/ijms25031375. Int J Mol Sci. 2024. PMID: 38338655 Free PMC article.
-
An Investigation of Nirmatrelvir (Paxlovid) Resistance in SARS-CoV-2 Mpro.ACS Bio Med Chem Au. 2024 Oct 8;4(6):280-290. doi: 10.1021/acsbiomedchemau.4c00045. eCollection 2024 Dec 18. ACS Bio Med Chem Au. 2024. PMID: 39712205 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

