Function Investigations and Applications of Membrane Proteins on Artificial Lipid Membranes
- PMID: 37108393
- PMCID: PMC10138308
- DOI: 10.3390/ijms24087231
Function Investigations and Applications of Membrane Proteins on Artificial Lipid Membranes
Abstract
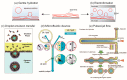
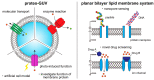
Membrane proteins play an important role in key cellular functions, such as signal transduction, apoptosis, and metabolism. Therefore, structural and functional studies of these proteins are essential in fields such as fundamental biology, medical science, pharmacology, biotechnology, and bioengineering. However, observing the precise elemental reactions and structures of membrane proteins is difficult, despite their functioning through interactions with various biomolecules in living cells. To investigate these properties, methodologies have been developed to study the functions of membrane proteins that have been purified from biological cells. In this paper, we introduce various methods for creating liposomes or lipid vesicles, from conventional to recent approaches, as well as techniques for reconstituting membrane proteins into artificial membranes. We also cover the different types of artificial membranes that can be used to observe the functions of reconstituted membrane proteins, including their structure, number of transmembrane domains, and functional type. Finally, we discuss the reconstitution of membrane proteins using a cell-free synthesis system and the reconstitution and function of multiple membrane proteins.
Keywords: artificial cell model; giant lipid vesicles; ion channels; liposome; membrane proteins; nanopores; planar bilayer lipid membrane; proteoliposome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology.Micromachines (Basel). 2020 May 30;11(6):559. doi: 10.3390/mi11060559. Micromachines (Basel). 2020. PMID: 32486297 Free PMC article. Review.
-
Formation of Giant Lipid Vesicle Containing Dual Functions Facilitates Outer Membrane Phospholipase.ACS Synth Biol. 2021 Aug 20;10(8):1837-1846. doi: 10.1021/acssynbio.0c00468. Epub 2021 Jul 14. ACS Synth Biol. 2021. PMID: 34258991
-
Tethered bilayer lipid membranes (tBLMs): interest and applications for biological membrane investigations.Biochimie. 2014 Dec;107 Pt A:135-42. doi: 10.1016/j.biochi.2014.06.021. Epub 2014 Jul 4. Biochimie. 2014. PMID: 24998327 Review.
-
Biopores/membrane proteins in synthetic polymer membranes.Biochim Biophys Acta Biomembr. 2017 Apr;1859(4):619-638. doi: 10.1016/j.bbamem.2016.10.015. Epub 2016 Oct 29. Biochim Biophys Acta Biomembr. 2017. PMID: 27984019 Review.
-
Membrane Structure-Function Insights from Asymmetric Lipid Vesicles.Acc Chem Res. 2019 Aug 20;52(8):2382-2391. doi: 10.1021/acs.accounts.9b00300. Epub 2019 Aug 6. Acc Chem Res. 2019. PMID: 31386337 Free PMC article.
Cited by
-
Synthetic control of actin polymerization and symmetry breaking in active protocells.Sci Adv. 2024 Jun 14;10(24):eadk9731. doi: 10.1126/sciadv.adk9731. Epub 2024 Jun 12. Sci Adv. 2024. PMID: 38865458 Free PMC article.
-
Advances in solubilization and stabilization techniques for structural and functional studies of membrane proteins.PeerJ. 2025 Apr 4;13:e19211. doi: 10.7717/peerj.19211. eCollection 2025. PeerJ. 2025. PMID: 40196297 Free PMC article. Review.
-
Artificial intelligence alphafold model for molecular biology and drug discovery: a machine-learning-driven informatics investigation.Mol Cancer. 2024 Oct 5;23(1):223. doi: 10.1186/s12943-024-02140-6. Mol Cancer. 2024. PMID: 39369244 Free PMC article.
-
Cell envelope diversity and evolution across the bacterial tree of life.Nat Microbiol. 2024 Oct;9(10):2475-2487. doi: 10.1038/s41564-024-01812-9. Epub 2024 Sep 18. Nat Microbiol. 2024. PMID: 39294462 Review.
-
Formation of Cell-Sized Liposomes Incorporating a β-Barrel-Structured Porin through Rehydration of a Phospholipid-Membrane Protein Dried Film.ACS Omega. 2024 Jan 26;9(5):5911-5918. doi: 10.1021/acsomega.3c09431. eCollection 2024 Feb 6. ACS Omega. 2024. PMID: 38343955 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

