Developmental Stage-Dependent Changes in Mitochondrial Function in the Brain of Offspring Following Prenatal Maternal Immune Activation
- PMID: 37108406
- PMCID: PMC10138707
- DOI: 10.3390/ijms24087243
Developmental Stage-Dependent Changes in Mitochondrial Function in the Brain of Offspring Following Prenatal Maternal Immune Activation
Abstract
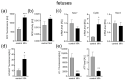
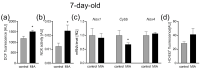
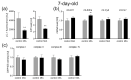
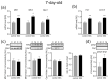
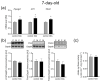
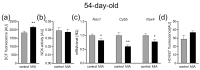
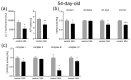
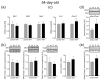
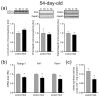
Maternal immune activation (MIA) is an important risk factor for neurodevelopmental disorders such as autism. The aim of the current study was to investigate the development-dependent changes in the mitochondrial function of MIA-exposed offspring, which may contribute to autism-like deficits. MIA was evoked by the single intraperitoneal administration of lipopolysaccharide to pregnant rats at gestation day 9.5, and several aspects of mitochondrial function in fetuses and in the brains of seven-day-old pups and adolescent offspring were analyzed along with oxidative stress parameters measurement. It was found that MIA significantly increased the activity of NADPH oxidase (NOX), an enzyme generating reactive oxygen species (ROS) in the fetuses and in the brain of seven-day-old pups, but not in the adolescent offspring. Although a lower mitochondrial membrane potential accompanied by a decreased ATP level was already observed in the fetuses and in the brain of seven-day-old pups, persistent alterations of ROS, mitochondrial membrane depolarization, and lower ATP generation with concomitant electron transport chain complexes downregulation were observed only in the adolescent offspring. We suggest that ROS observed in infancy are most likely of a NOX activity origin, whereas in adolescence, ROS are produced by damaged mitochondria. The accumulation of dysfunctional mitochondria leads to the intense release of free radicals that trigger oxidative stress and neuroinflammation, resulting in an interlinked vicious cascade.
Keywords: NADPH oxidase; ROS; animal model; autism; maternal immune activation; mitochondria; neurodevelopmental disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model.Brain Behav Immun. 2014 Nov;42:138-46. doi: 10.1016/j.bbi.2014.06.013. Epub 2014 Jun 26. Brain Behav Immun. 2014. PMID: 24973728
-
The Synaptic Dysregulation in Adolescent Rats Exposed to Maternal Immune Activation.Front Mol Neurosci. 2021 Jan 14;13:555290. doi: 10.3389/fnmol.2020.555290. eCollection 2020. Front Mol Neurosci. 2021. PMID: 33519375 Free PMC article.
-
Gestational exposure to a viral mimetic poly(i:C) results in long-lasting changes in mitochondrial function by leucocytes in the adult offspring.Mediators Inflamm. 2013;2013:609602. doi: 10.1155/2013/609602. Epub 2013 Sep 19. Mediators Inflamm. 2013. PMID: 24174710 Free PMC article.
-
The Role of Maternal Immune Activation in the Pathogenesis of Autism: A Review of the Evidence, Proposed Mechanisms and Implications for Treatment.Int J Mol Sci. 2021 Oct 26;22(21):11516. doi: 10.3390/ijms222111516. Int J Mol Sci. 2021. PMID: 34768946 Free PMC article. Review.
-
Maternal Inflammation During Pregnancy and Offspring Brain Development: The Role of Mitochondria.Biol Psychiatry Cogn Neurosci Neuroimaging. 2022 May;7(5):498-509. doi: 10.1016/j.bpsc.2021.11.003. Epub 2021 Nov 17. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022. PMID: 34800727 Free PMC article. Review.
Cited by
-
Prenatal modulation of NADPH-oxidase reverses the deranged GABA switch and rescues behavioral deficits in valproate ASD rat model.Front Pharmacol. 2025 May 30;16:1571008. doi: 10.3389/fphar.2025.1571008. eCollection 2025. Front Pharmacol. 2025. PMID: 40520207 Free PMC article.
-
IL-6 Enhances the Activation of PI3K-AKT/mTOR-GSK-3β by Upregulating GRPR in Hippocampal Neurons of Autistic Mice.J Neuroimmune Pharmacol. 2024 Mar 27;19(1):12. doi: 10.1007/s11481-024-10111-3. J Neuroimmune Pharmacol. 2024. PMID: 38536552 Free PMC article.
-
The Role of Purine Metabolism and Uric Acid in Postnatal Neurologic Development.Molecules. 2025 Feb 11;30(4):839. doi: 10.3390/molecules30040839. Molecules. 2025. PMID: 40005150 Free PMC article. Review.
-
Prenatal immune activation in mice induces long-term alterations in brain mitochondrial function.Transl Psychiatry. 2024 Jul 16;14(1):289. doi: 10.1038/s41398-024-03010-x. Transl Psychiatry. 2024. PMID: 39009558 Free PMC article.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Washington, DC, USA: 2013. pp. 591–643. - DOI
-
- Zuckerman L., Rehavi M., Nachman R., Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: A novel neurodevelopmental model of schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

