Reorganization and Suppression of Store-Operated Calcium Entry in Podocytes of Type 2 Diabetic Rats
- PMID: 37108424
- PMCID: PMC10139047
- DOI: 10.3390/ijms24087259
Reorganization and Suppression of Store-Operated Calcium Entry in Podocytes of Type 2 Diabetic Rats
Abstract
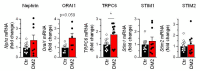
Type 2 diabetes mellitus (DM2) is a widespread metabolic disorder that results in podocyte damage and diabetic nephropathy. Previous studies demonstrated that TRPC6 channels play a pivotal role in podocyte function and their dysregulation is associated with development of different kidney diseases including nephropathy. Here, using single channel patch clamp technique, we demonstrated that non-selective cationic TRPC6 channels are sensitive to the Ca2+ store depletion in human podocyte cell line Ab8/13 and in freshly isolated rat glomerular podocytes. Ca2+ imaging indicated the involvement of ORAI and sodium-calcium exchanger in Ca2+ entry induced upon store depletion. In male rats fed a high-fat diet combined with a low-dose streptozotocin injection, which leads to DM2 development, we observed the reduction of a store-operated Ca2+ entry (SOCE) in rat glomerular podocytes. This was accompanied by a reorganization of store-operated Ca2+ influx such that TRPC6 channels lost their sensitivity to Ca2+ store depletion and ORAI-mediated Ca2+ entry was suppressed in TRPC6-independent manner. Altogether our data provide new insights into the mechanism of SOCE organization in podocytes in the norm and in pathology, which should be taken into account when developing pharmacological treatment of the early stages of diabetic nephropathy.
Keywords: ORAI channels; TRPC6 channels; diabetic nephropathy; sodium–calcium exchanger (NCX); store-operated calcium entry (SOCE); type 2 diabetes mellitus.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

