Dexamethasone Modulates the Cytokine Response but Not COVID-19-Induced Coagulopathy in Critically Ill
- PMID: 37108440
- PMCID: PMC10138864
- DOI: 10.3390/ijms24087278
Dexamethasone Modulates the Cytokine Response but Not COVID-19-Induced Coagulopathy in Critically Ill
Abstract
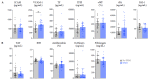
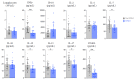
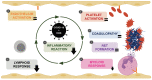
Severe forms of coronavirus 2019 (COVID-19) disease are caused by an exaggerated systemic inflammatory response and subsequent inflammation-related coagulopathy. Anti-inflammatory treatment with low dose dexamethasone has been shown to reduce mortality in COVID-19 patients requiring oxygen therapy. However, the mechanisms of action of corticosteroids have not been extensively studied in critically ill patients in the context of COVID-19. Plasma biomarkers of inflammatory and immune responses, endothelial and platelet activation, neutrophil extracellular trap formation, and coagulopathy were compared between patients treated or not by systemic dexamethasone for severe forms of COVID-19. Dexamethasone treatment significantly reduced the inflammatory and lymphoid immune response in critical COVID-19 patients but had little effect on the myeloid immune response and no effect on endothelial activation, platelet activation, neutrophil extracellular trap formation, and coagulopathy. The benefits of low dose dexamethasone on outcome in critical COVID-19 can be partially explained by a modulation of the inflammatory response but not by reduction of coagulopathy. Future studies should explore the impact of combining dexamethasone with other immunomodulatory or anticoagulant drugs in severe COVID-19.
Keywords: COVID-19; coagulopathy; cytokine storm; inflammation; low dose dexamethasone.
Conflict of interest statement
This work was supported by grants from the Foundation Saint-Luc (Brussels, Belgium). The Division of Cardiology at Cliniques Universitaires Saint-Luc, Belgium, has received unrestricted research grants from AstraZeneca (Belgium). MD is Clinical Master Specialist Applicant to a Ph.D. at the Fonds National de la Recherche Scientifique et Médicale (FNRS, Belgium). JDP was supported by a grant from the Salus Sanguinis Foundation (UCLouvain, Belgium). MO, LP, and JB are supported by Fund for Research Training in Industry and Agriculture (FRIA, FNRS). LB and SH are senior research associates at FNRS. QUALIblood s.a. offered the analyses for determining the cytokine profile.
Figures



References
-
- Morris G., Bortolasci C.C., Puri B.K., Marx W., O’Neil A., Athan E., Walder K., Berk M., Olive L., Carvalho A.F., et al. The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all? Cytokine. 2021;144:155593. doi: 10.1016/j.cyto.2021.155593. - DOI - PMC - PubMed
-
- Dechamps M., De Poortere J., Martin M., Gatto L., Daumerie A., Bouzin C., Octave M., Ginion A., Robaux V., Pirotton L., et al. Inflammation-Induced Coagulopathy Substantially Differs Between COVID-19 and Septic Shock: A Prospective Observational Study. Front. Med. 2022;8:3099. doi: 10.3389/fmed.2021.780750. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

