Dynamic Changes of BVRA Protein Levels Occur in Response to Insulin: A Pilot Study in Humans
- PMID: 37108445
- PMCID: PMC10138944
- DOI: 10.3390/ijms24087282
Dynamic Changes of BVRA Protein Levels Occur in Response to Insulin: A Pilot Study in Humans
Abstract
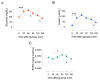
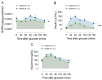
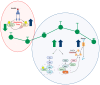
Biliverdin reductase-A (BVRA) is involved in the regulation of insulin signaling and the maintenance of glucose homeostasis. Previous research showed that BVRA alterations are associated with the aberrant activation of insulin signaling in dysmetabolic conditions. However, whether BVRA protein levels change dynamically within the cells in response to insulin and/or glucose remains an open question. To this aim, we evaluated changes of intracellular BVRA levels in peripheral blood mononuclear cells (PBMC) collected during the oral glucose tolerance test (OGTT) in a group of subjects with different levels of insulin sensitivity. Furthermore, we looked for significant correlations with clinical measures. Our data show that BVRA levels change dynamically during the OGTT in response to insulin, and greater BVRA variations occur in those subjects with lower insulin sensitivity. Changes of BVRA significantly correlate with indexes of increased insulin resistance and insulin secretion (HOMA-IR, HOMA-β, and insulinogenic index). At the multivariate regression analysis, the insulinogenic index independently predicted increased BVRA area under curve (AUC) during the OGTT. This pilot study showed, for the first time, that intracellular BVRA protein levels change in response to insulin during OGTT and are greater in subjects with lower insulin sensitivity, supporting the role of BVR-A in the dynamic regulation of the insulin signaling pathway.
Keywords: biliverdin reductase-A; diabetes; insulin signaling; metabolism; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Triani F., Tramutola A., Di Domenico F., Sharma N., Butterfield D.A., Head E., Perluigi M., Barone E. Biliverdin reductase-A impairment links brain insulin resistance with increased Abeta production in an animal model of aging: Implications for Alzheimer disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3181–3194. doi: 10.1016/j.bbadis.2018.07.005. - DOI - PubMed
-
- Gibbs P.E., Miralem T., Lerner-Marmarosh N., Tudor C., Maines M.D. Formation of ternary complex of human biliverdin reductase-protein kinase Cdelta-ERK2 protein is essential for ERK2-mediated activation of Elk1 protein, nuclear factor-kappaB, and inducible nitric-oxidase synthase (iNOS) J. Biol. Chem. 2012;287:1066–1079. doi: 10.1074/jbc.M111.279612. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

