An Efficient 2D Protocol for Differentiation of iPSCs into Mature Postmitotic Dopaminergic Neurons: Application for Modeling Parkinson's Disease
- PMID: 37108456
- PMCID: PMC10139404
- DOI: 10.3390/ijms24087297
An Efficient 2D Protocol for Differentiation of iPSCs into Mature Postmitotic Dopaminergic Neurons: Application for Modeling Parkinson's Disease
Abstract
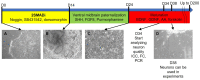
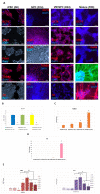
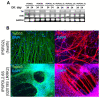
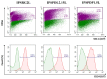
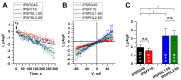
About 15% of patients with parkinsonism have a hereditary form of Parkinson's disease (PD). Studies on the early stages of PD pathogenesis are challenging due to the lack of relevant models. The most promising ones are models based on dopaminergic neurons (DAns) differentiated from induced pluripotent stem cells (iPSCs) of patients with hereditary forms of PD. This work describes a highly efficient 2D protocol for obtaining DAns from iPSCs. The protocol is rather simple, comparable in efficiency with previously published protocols, and does not require viral vectors. The resulting neurons have a similar transcriptome profile to previously published data for neurons, and have a high level of maturity marker expression. The proportion of sensitive (SOX6+) DAns in the population calculated from the level of gene expression is higher than resistant (CALB+) DAns. Electrophysiological studies of the DAns confirmed their voltage sensitivity and showed that a mutation in the PARK8 gene is associated with enhanced store-operated calcium entry. The study of high-purity DAns differentiated from the iPSCs of patients with hereditary PD using this differentiation protocol will allow for investigators to combine various research methods, from patch clamp to omics technologies, and maximize information about cell function in normal and pathological conditions.
Keywords: Parkinson’s disease; SOCE currents; calcium; differentiation protocol; disease modeling; high purity of neuron culture; iPSCs.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Generation of Naivetropic Induced Pluripotent Stem Cells from Parkinson's Disease Patients for High-Efficiency Genetic Manipulation and Disease Modeling.Stem Cells Dev. 2015 Nov 1;24(21):2591-604. doi: 10.1089/scd.2015.0079. Epub 2015 Sep 1. Stem Cells Dev. 2015. PMID: 26218671 Free PMC article.
-
Synthetic mRNAs Drive Highly Efficient iPS Cell Differentiation to Dopaminergic Neurons.Stem Cells Transl Med. 2019 Feb;8(2):112-123. doi: 10.1002/sctm.18-0036. Epub 2018 Nov 1. Stem Cells Transl Med. 2019. PMID: 30387318 Free PMC article.
-
TREM2 gene induces differentiation of induced pluripotent stem cells into dopaminergic neurons and promotes neuronal repair via TGF-β activation in 6-OHDA-lesioned mouse model of Parkinson's disease.CNS Neurosci Ther. 2024 Feb;30(2):e14630. doi: 10.1111/cns.14630. CNS Neurosci Ther. 2024. PMID: 38348765 Free PMC article.
-
Cellular reprogramming: a new approach to modelling Parkinson's disease.Biochem Soc Trans. 2012 Oct;40(5):1152-7. doi: 10.1042/BST20120159. Biochem Soc Trans. 2012. PMID: 22988881 Review.
-
Treatment of Parkinson's Disease through Personalized Medicine and Induced Pluripotent Stem Cells.Cells. 2019 Jan 7;8(1):26. doi: 10.3390/cells8010026. Cells. 2019. PMID: 30621042 Free PMC article. Review.
Cited by
-
SinCMat: A single-cell-based method for predicting functional maturation transcription factors.Stem Cell Reports. 2024 Feb 13;19(2):270-284. doi: 10.1016/j.stemcr.2023.12.006. Epub 2024 Jan 11. Stem Cell Reports. 2024. PMID: 38215756 Free PMC article.
-
GQIcombi application to subdue glioma via differentiation therapy.Front Oncol. 2024 Jun 26;14:1322795. doi: 10.3389/fonc.2024.1322795. eCollection 2024. Front Oncol. 2024. PMID: 38988707 Free PMC article.
-
STIM Proteins: The Gas and Brake of Calcium Entry in Neurons.Neurosci Bull. 2025 Feb;41(2):305-325. doi: 10.1007/s12264-024-01272-5. Epub 2024 Sep 12. Neurosci Bull. 2025. PMID: 39266936 Review.
-
Metabolic Culture Medium Enhances Maturation of Human iPSC-Derived Cardiomyocytes via Cardiac Troponin I Isoform Induction.Int J Mol Sci. 2025 Jul 26;26(15):7248. doi: 10.3390/ijms26157248. Int J Mol Sci. 2025. PMID: 40806381 Free PMC article.
References
-
- Sudhaman S., Prasad K., Behari M., Muthane U.B., Juyal R.C., Thelma B. Discovery of a frameshift mutation in podocalyxin-like (PODXL) gene, coding for a neural adhesion molecule, as causal for autosomal-recessive juvenile Parkinsonism. J. Med. Genet. 2016;53:450–456. doi: 10.1136/jmedgenet-2015-103459. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

