Altered Brain Expression of DNA Methylation and Hydroxymethylation Epigenetic Enzymes in a Rat Model of Neuropathic Pain
- PMID: 37108466
- PMCID: PMC10138521
- DOI: 10.3390/ijms24087305
Altered Brain Expression of DNA Methylation and Hydroxymethylation Epigenetic Enzymes in a Rat Model of Neuropathic Pain
Abstract
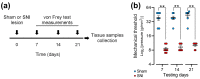
The role of epigenetics in chronic pain at the supraspinal level is yet to be fully characterized. DNA histone methylation is crucially regulated by de novo methyltransferases (DNMT1-3) and ten-eleven translocation dioxygenases (TET1-3). Evidence has shown that methylation markers are altered in different CNS regions related to nociception, namely the dorsal root ganglia, the spinal cord, and different brain areas. Decreased global methylation was found in the DRG, the prefrontal cortex, and the amygdala, which was associated with decreased DNMT1/3a expression. In contrast, increased methylation levels and mRNA levels of TET1 and TET3 were linked to augmented pain hypersensitivity and allodynia in inflammatory and neuropathic pain models. Since epigenetic mechanisms may be responsible for the regulation and coordination of various transcriptional modifications described in chronic pain states, with this study, we aimed to evaluate the functional role of TET1-3 and DNMT1/3a genes in neuropathic pain in several brain areas. In a spared nerve injury rat model of neuropathic pain, 21 days after surgery, we found increased TET1 expression in the medial prefrontal cortex and decreased expression in the caudate-putamen and the amygdala; TET2 was upregulated in the medial thalamus; TET3 mRNA levels were reduced in the medial prefrontal cortex and the caudate-putamen; and DNMT1 was downregulated in the caudate-putamen and the medial thalamus. No statistically significant changes in expression were observed with DNMT3a. Our results suggest a complex functional role for these genes in different brain areas in the context of neuropathic pain. The notion of DNA methylation and hydroxymethylation being cell-type specific and not tissue specific, as well as the possibility of chronologically differential gene expression after the establishment of neuropathic or inflammatory pain models, ought to be addressed in future studies.
Keywords: SNI; brain; epigenetics; gene expression; neuropathic pain; pain; rat model.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Dahlhamer J., Lucas J., Zelaya C., Nahin R., Mackey S., DeBar L., Kerns R., Von Korff M., Porter L., Helmick C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018;67:1001–1006. doi: 10.15585/mmwr.mm6736a2. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

