ADAM10 and ADAM17, Major Regulators of Chronic Kidney Disease Induced Atherosclerosis?
- PMID: 37108478
- PMCID: PMC10139114
- DOI: 10.3390/ijms24087309
ADAM10 and ADAM17, Major Regulators of Chronic Kidney Disease Induced Atherosclerosis?
Abstract
Chronic kidney disease (CKD) is a major health problem, affecting millions of people worldwide, in particular hypertensive and diabetic patients. CKD patients suffer from significantly increased cardiovascular disease (CVD) morbidity and mortality, mainly due to accelerated atherosclerosis development. Indeed, CKD not only affects the kidneys, in which injury and maladaptive repair processes lead to local inflammation and fibrosis, but also causes systemic inflammation and altered mineral bone metabolism leading to vascular dysfunction, calcification, and thus, accelerated atherosclerosis. Although CKD and CVD individually have been extensively studied, relatively little research has studied the link between both diseases. This narrative review focuses on the role of a disintegrin and metalloproteases (ADAM) 10 and ADAM17 in CKD and CVD and will for the first time shed light on their role in CKD-induced CVD. By cleaving cell surface molecules, these enzymes regulate not only cellular sensitivity to their micro-environment (in case of receptor cleavage), but also release soluble ectodomains that can exert agonistic or antagonistic functions, both locally and systemically. Although the cell-specific roles of ADAM10 and ADAM17 in CVD, and to a lesser extent in CKD, have been explored, their impact on CKD-induced CVD is likely, yet remains to be elucidated.
Keywords: a disintegrin and metalloprotease; atherosclerosis; cardiovascular diseases; chronic kidney disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
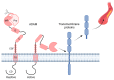
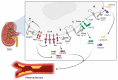
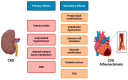
References
-
- World Health Organization Mortality and Global Health Estimates. [(accessed on 16 January 2023)]. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-est....
-
- National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–S266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

