Inhibition of ROS-Scavenging Enzyme System Is a Key Event in Tomato Genetic Resistance against Root-Knot Nematodes
- PMID: 37108485
- PMCID: PMC10138560
- DOI: 10.3390/ijms24087324
Inhibition of ROS-Scavenging Enzyme System Is a Key Event in Tomato Genetic Resistance against Root-Knot Nematodes
Abstract
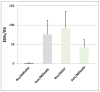
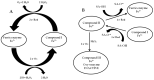
Genetic resistance in plants against incompatible pests is expressed by the activation of an immune system; however, the molecular mechanisms of pest recognition and expression of immunity, although long the object of investigation, are far from being fully understood. The immune response triggered by the infection of soil-borne parasites, such as root-knot nematodes (RKNs), to incompatible resistant tomato plants was studied and compared to the compatible response that occurred when RKNs attacked susceptible plants. In compatible interactions, the invading nematode juveniles were allowed to fully develop and reproduce, whilst that was impeded in incompatible interactions. In crude root extracts, a first assay of reactive oxygen species (ROS)-scavenging enzymatic activity was carried out at the earliest stages of tomato-RKN incompatible interaction. Membrane-bound and soluble CAT, which is the most active enzyme in hydrogen peroxide (H2O2) scavenging, was found to be specifically inhibited in roots of inoculated resistant plants until 5 days after inoculation, with respect to uninoculated plants. The expression of genes encoding for antioxidant enzymes, such as CAT and glutathione peroxidase (GPX), was not always inhibited in roots of nematode-infected resistant tomato. Therefore, the biochemical mechanisms of CAT inhibition were further investigated. Two CAT isozymes were characterized by size exclusion HPLC as a tetrameric form with a molecular weight of 220,000 dalton and its subunits (55,000 dalton). Fractions containing such isozymes were tested by their sensitivity to both salicylic acid (SA) and H2O2. It was evidenced that elevated concentrations of both chemicals led to a partial inactivation of CAT. Elevated concentrations of H2O2 in incompatible interactions have been suggested to be produced by membrane-bound superoxide anion generating, SOD, and isoperoxidase-enhanced activities. Such partial inactivation of CAT has been depicted as one of the earliest key metabolic events, which is specifically associated with tomato immunity to RKNs. Enhanced ROS production and the inhibition of ROS-scavenging systems have been considered to trigger all the metabolic events leading to cell death and tissue necrosis developed around the head of the invading juveniles by which this special type of plant resistance is exerted.
Keywords: RBOHD; ROS; antioxidants; catalase; hydrogen peroxide; pest resistance; root-knot nematodes; superoxide anions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Root-Knot Nematode Early Infection Suppresses Immune Response and Elicits the Antioxidant System in Tomato.Int J Mol Sci. 2024 Nov 23;25(23):12602. doi: 10.3390/ijms252312602. Int J Mol Sci. 2024. PMID: 39684315 Free PMC article.
-
Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes.PLoS One. 2019 Dec 3;14(12):e0213230. doi: 10.1371/journal.pone.0213230. eCollection 2019. PLoS One. 2019. PMID: 31794550 Free PMC article.
-
ROS and Cell Death in Tomato Roots Infected by Meloidogyne Incognita.Methods Mol Biol. 2018;1743:87-95. doi: 10.1007/978-1-4939-7668-3_8. Methods Mol Biol. 2018. PMID: 29332288
-
Redox signalling in plant-nematode interactions: Insights into molecular crosstalk and defense mechanisms.Plant Cell Environ. 2024 Aug;47(8):2811-2820. doi: 10.1111/pce.14925. Epub 2024 Apr 28. Plant Cell Environ. 2024. PMID: 38679939 Review.
-
Rooting Out the Mechanisms of Root-Knot Nematode-Plant Interactions.Annu Rev Phytopathol. 2022 Aug 26;60:43-76. doi: 10.1146/annurev-phyto-021621-120943. Epub 2022 Mar 22. Annu Rev Phytopathol. 2022. PMID: 35316614 Review.
Cited by
-
Root-Knot Nematode Early Infection Suppresses Immune Response and Elicits the Antioxidant System in Tomato.Int J Mol Sci. 2024 Nov 23;25(23):12602. doi: 10.3390/ijms252312602. Int J Mol Sci. 2024. PMID: 39684315 Free PMC article.
-
Biochemical Defence of Plants against Parasitic Nematodes.Plants (Basel). 2024 Oct 8;13(19):2813. doi: 10.3390/plants13192813. Plants (Basel). 2024. PMID: 39409684 Free PMC article. Review.
-
The molecular dynamics between reactive oxygen species (ROS), reactive nitrogen species (RNS) and phytohormones in plant's response to biotic stress.Plant Cell Rep. 2024 Oct 16;43(11):263. doi: 10.1007/s00299-024-03343-3. Plant Cell Rep. 2024. PMID: 39412663 Review.
References
-
- Molinari S. Can the plant immune system be activated against animal parasites such as nematodes? Nematology. 2020;22:1–12. doi: 10.1163/15685411-bja10026. - DOI
-
- Milligan S.B., Bodeau J., Yaghoobi J., Kaloshian I., Zabel P., Williamson V.M. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell. 1998;10:1307–1319. doi: 10.1105/tpc.10.8.1307. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

