Evidence of the Dysbiotic Effect of Psychotropics on Gut Microbiota and Capacity of Probiotics to Alleviate Related Dysbiosis in a Model of the Human Colon
- PMID: 37108487
- PMCID: PMC10138884
- DOI: 10.3390/ijms24087326
Evidence of the Dysbiotic Effect of Psychotropics on Gut Microbiota and Capacity of Probiotics to Alleviate Related Dysbiosis in a Model of the Human Colon
Abstract
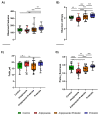
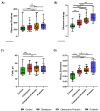
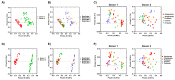
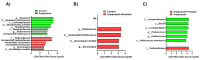
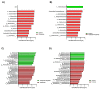
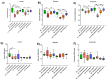
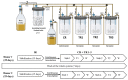
Growing evidence indicates that non-antibiotic therapeutics significantly impact human health by modulating gut microbiome composition and metabolism. In this study, we investigated the impact of two psychotropic drugs, aripiprazole and (S)-citalopram, on gut microbiome composition and its metabolic activity, as well as the potential of probiotics to attenuate related dysbiosis using an ex vivo model of the human colon. After 48 h of fermentation, the two psychotropics demonstrated distinct modulatory effects on the gut microbiome. Aripiprazole, at the phylum level, significantly decreased the relative abundances of Firmicutes and Actinobacteria, while increasing the proportion of Proteobacteria. Moreover, the families Lachnospiraceae, Lactobacillaceae, and Erysipelotrichaceae were also reduced by aripiprazole treatment compared to the control group. In addition, aripiprazole lowered the levels of butyrate, propionate, and acetate, as measured by gas chromatography (GC). On the other hand, (S)-citalopram increased the alpha diversity of microbial taxa, with no differences observed between groups at the family and genus level. Furthermore, a probiotic combination of Lacticaseibacillus rhamnosus HA-114 and Bifidobacterium longum R0175 alleviated gut microbiome alterations and increased the production of short-chain fatty acids to a similar level as the control. These findings provide compelling evidence that psychotropics modulate the composition and function of the gut microbiome, while the probiotic can mitigate related dysbiosis.
Keywords: (S)-citalopram; aripiprazole; gut dysbiosis; gut microbiome; probiotics; psychotropics.
Conflict of interest statement
T.A.T. is an employee of Lallemand Bio-Ingredients. The remaining authors report no conflict of interest.
Figures
Similar articles
-
Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients.Nutrients. 2023 Jan 11;15(2):356. doi: 10.3390/nu15020356. Nutrients. 2023. PMID: 36678227 Free PMC article. Clinical Trial.
-
Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function.Psychopharmacology (Berl). 2019 May;236(5):1671-1685. doi: 10.1007/s00213-018-5006-5. Epub 2018 Aug 28. Psychopharmacology (Berl). 2019. PMID: 30155748
-
Beneficial effects of probiotics on dysbiosis of gut microbiota induced by antibiotic treatment in healthy dogs.Res Vet Sci. 2025 Aug;191:105674. doi: 10.1016/j.rvsc.2025.105674. Epub 2025 May 9. Res Vet Sci. 2025. PMID: 40347600
-
Nutritional and therapeutic approaches for protecting human gut microbiota from psychotropic treatments.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Jun 8;108:110182. doi: 10.1016/j.pnpbp.2020.110182. Epub 2020 Nov 21. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 33232785 Review.
-
Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies.Crit Rev Food Sci Nutr. 2022;62(6):1427-1452. doi: 10.1080/10408398.2020.1843396. Epub 2020 Nov 16. Crit Rev Food Sci Nutr. 2022. PMID: 33198506 Review.
Cited by
-
Pancreatitis affects gut microbiota via metabolites and inflammatory cytokines: an exploratory two-step Mendelian randomisation study.Mol Genet Genomics. 2024 Mar 16;299(1):36. doi: 10.1007/s00438-024-02125-6. Mol Genet Genomics. 2024. PMID: 38492113 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

