Novel Functionalized Spiro [Indoline-3,5'-pyrroline]-2,2'dione Derivatives: Synthesis, Characterization, Drug-Likeness, ADME, and Anticancer Potential
- PMID: 37108498
- PMCID: PMC10139052
- DOI: 10.3390/ijms24087336
Novel Functionalized Spiro [Indoline-3,5'-pyrroline]-2,2'dione Derivatives: Synthesis, Characterization, Drug-Likeness, ADME, and Anticancer Potential
Abstract
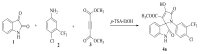
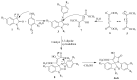
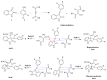
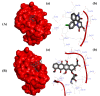
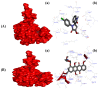
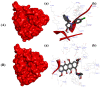
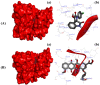
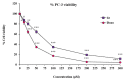
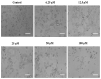
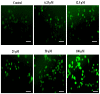
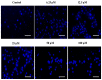
A highly stereo-selective, one-pot, multicomponent method was chosen to synthesize the novel functionalized 1, 3-cycloaddition spirooxindoles (SOXs) (4a-4h). Synthesized SOXs were analyzed for their drug-likeness and ADME parameters and screened for their anticancer activity. Our molecular docking analysis revealed that among all derivatives of SOXs (4a-4h), 4a has a substantial binding affinity (∆G) -6.65, -6.55, -8.73, and -7.27 Kcal/mol with CD-44, EGFR, AKR1D1, and HER-2, respectively. A functional study demonstrated that SOX 4a has a substantial impact on human cancer cell phenotypes exhibiting abnormality in cytoplasmic and nuclear architecture as well as granule formation leading to cell death. SOX 4a treatment robustly induced reactive oxygen species (ROS) generation in cancer cells as observed by enhanced DCFH-DA signals. Overall, our results suggest that SOX (4a) targets CD-44, EGFR, AKR1D1, and HER-2 and induces ROS generation in cancer cells. We conclude that SOX (4a) could be explored as a potential chemotherapeutic molecule against various cancers in appropriate pre-clinical in vitro and in vivo model systems.
Keywords: ADMET studies; ROS generation; and anticancer effects; cancer cells; drug-likeness; isatin-derived spirooxindoles (SOXs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A green one-pot synthetic protocol of hexahydropyrimido[4,5-d]pyrimidin-4(1H)-one derivatives: molecular docking, ADMET, anticancer and antimicrobial studies.Mol Divers. 2024 Feb;28(1):183-195. doi: 10.1007/s11030-023-10712-9. Epub 2023 Aug 11. Mol Divers. 2024. PMID: 37566199
-
New pyrimidine and pyrazole-based compounds as potential EGFR inhibitors: Synthesis, anticancer, antimicrobial evaluation and computational studies.Bioorg Chem. 2021 Sep;114:105078. doi: 10.1016/j.bioorg.2021.105078. Epub 2021 Jun 10. Bioorg Chem. 2021. PMID: 34161878
-
Chemical Characterization, In-silico Evaluation, and Molecular Docking Analysis of Antiproliferative Compounds Isolated from the Bark of Anthocephalus cadamba Miq.Anticancer Agents Med Chem. 2022;22(20):3416-3437. doi: 10.2174/1871520622666220204123348. Anticancer Agents Med Chem. 2022. PMID: 35125087
-
EGFR/VEGFR-2 dual inhibitor and apoptotic inducer: Design, synthesis, anticancer activity and docking study of new 2-thioxoimidazolidin-4one derivatives.Life Sci. 2021 Jul 15;277:119531. doi: 10.1016/j.lfs.2021.119531. Epub 2021 Apr 21. Life Sci. 2021. PMID: 33887348
-
Novel benzothiazole-based dual VEGFR-2/EGFR inhibitors targeting breast and liver cancers: Synthesis, cytotoxic activity, QSAR and molecular docking studies.Bioorg Med Chem Lett. 2022 Feb 15;58:128529. doi: 10.1016/j.bmcl.2022.128529. Epub 2022 Jan 7. Bioorg Med Chem Lett. 2022. PMID: 35007724 Review.
Cited by
-
Honey Targets Ribosome Biogenesis Components to Suppress the Growth of Human Pancreatic Cancer Cells.Cancers (Basel). 2024 Oct 9;16(19):3431. doi: 10.3390/cancers16193431. Cancers (Basel). 2024. PMID: 39410048 Free PMC article.
-
Exploring the Therapeutic Potential of Petiveria alliacea L. Phytochemicals: A Computational Study on Inhibiting SARS-CoV-2's Main Protease (Mpro).Molecules. 2024 May 27;29(11):2524. doi: 10.3390/molecules29112524. Molecules. 2024. PMID: 38893400 Free PMC article.
-
Inulin-based formulations as an emerging therapeutic strategy for cancer: A comprehensive review.Int J Biol Macromol. 2024 Feb;259(Pt 1):129216. doi: 10.1016/j.ijbiomac.2024.129216. Epub 2024 Jan 5. Int J Biol Macromol. 2024. PMID: 38185294 Free PMC article. Review.
-
Quantum chemical modeling, molecular docking, and ADMET evaluation of imidazole phenothiazine hybrids.Sci Rep. 2025 Jul 2;15(1):23413. doi: 10.1038/s41598-025-90495-1. Sci Rep. 2025. PMID: 40604198 Free PMC article.
References
-
- International Agency for Research on Cancer Prostate . Source: Globocan 2020 Number of New Cases in 2020, Both Sexes, All Ages. IARC; Lyon, France: 2020.
-
- Patrawala L., Calhoun T., Schneider-Broussard R., Li H., Bhatia B., Tang S., Reilly J.G., Chandra D., Zhou J., Claypool K., et al. Highly Purified CD44+ Prostate Cancer Cells from Xenograft Human Tumors Are Enriched in Tumorigenic and Metastatic Progenitor Cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

