G-Protein β-Subunit Gene TaGB1-B Enhances Drought and Salt Resistance in Wheat
- PMID: 37108500
- PMCID: PMC10138664
- DOI: 10.3390/ijms24087337
G-Protein β-Subunit Gene TaGB1-B Enhances Drought and Salt Resistance in Wheat
Abstract
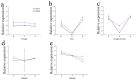
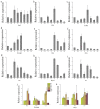
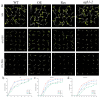
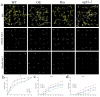
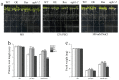
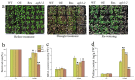
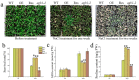
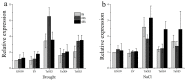
In the hexaploid wheat genome, there are three Gα genes, three Gβ and twelve Gγ genes, but the function of Gβ in wheat has not been explored. In this study, we obtained the overexpression of TaGB1 Arabidopsis plants through inflorescence infection, and the overexpression of wheat lines was obtained by gene bombardment. The results showed that under drought and NaCl treatment, the survival rate of Arabidopsis seedlings' overexpression of TaGB1-B was higher than that of the wild type, while the survival rate of the related mutant agb1-2 was lower than that of the wild type. The survival rate of wheat seedlings with TaGB1-B overexpression was higher than that of the control. In addition, under drought and salt stress, the levels of superoxide dismutase (SOD) and proline (Pro) in the wheat overexpression of TaGB1-B were higher than that of the control, and the concentration of malondialdehyde (MDA) was lower than that of the control. This indicates that TaGB1-B could improve the drought resistance and salt tolerance of Arabidopsis and wheat by scavenging active oxygen. Overall, this work provides a theoretical basis for wheat G-protein β-subunits in a further study, and new genetic resources for the cultivation of drought-tolerant and salt-tolerant wheat varieties.
Keywords: G-protein β-subunit; TaGB1; abiotic stress; wheat.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Wheat TaTIP4;1 Confers Enhanced Tolerance to Drought, Salt and Osmotic Stress in Arabidopsis and Rice.Int J Mol Sci. 2022 Feb 14;23(4):2085. doi: 10.3390/ijms23042085. Int J Mol Sci. 2022. PMID: 35216200 Free PMC article.
-
TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis.BMC Plant Biol. 2015 Nov 4;15:268. doi: 10.1186/s12870-015-0644-9. BMC Plant Biol. 2015. PMID: 26536863 Free PMC article.
-
A wheat lipid transfer protein (TdLTP4) promotes tolerance to abiotic and biotic stress in Arabidopsis thaliana.Plant Physiol Biochem. 2015 Apr;89:64-75. doi: 10.1016/j.plaphy.2015.02.008. Epub 2015 Feb 14. Plant Physiol Biochem. 2015. PMID: 25703105
-
The wheat VQ motif-containing protein TaVQ4-D positively regulates drought tolerance in transgenic plants.J Exp Bot. 2023 Sep 29;74(18):5591-5605. doi: 10.1093/jxb/erad280. J Exp Bot. 2023. PMID: 37471263
-
Wheat WRKY transcription factor TaWRKY24 confers drought and salt tolerance in transgenic plants.Plant Physiol Biochem. 2023 Dec;205:108137. doi: 10.1016/j.plaphy.2023.108137. Epub 2023 Oct 24. Plant Physiol Biochem. 2023. PMID: 37977027
Cited by
-
Salinity survival: molecular mechanisms and adaptive strategies in plants.Front Plant Sci. 2025 Feb 28;16:1527952. doi: 10.3389/fpls.2025.1527952. eCollection 2025. Front Plant Sci. 2025. PMID: 40093605 Free PMC article. Review.
-
Genome-Wide Identification of the GS3 Gene Family and the Influence of Natural Variations in BnGS3-3 on Salt and Cold Stress Tolerance in Brassica napus.Plants (Basel). 2025 Apr 7;14(7):1145. doi: 10.3390/plants14071145. Plants (Basel). 2025. PMID: 40219212 Free PMC article.
-
Genetic Basis of Grain Size and Weight in Rice, Wheat, and Barley.Int J Mol Sci. 2023 Nov 29;24(23):16921. doi: 10.3390/ijms242316921. Int J Mol Sci. 2023. PMID: 38069243 Free PMC article. Review.
-
Genome-wide identification of heterotrimeric G protein genes in castor (Ricinus communis L.) and expression patterns under salt stress.BMC Genomics. 2024 Nov 20;25(1):1115. doi: 10.1186/s12864-024-11027-1. BMC Genomics. 2024. PMID: 39567878 Free PMC article.
References
-
- Shao H., Liang Z., Shao M. Adaptation of higher plants to environmental stresses and stress signal transduction. Acta Ecol. Sin. 2005;25:1772–1781.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

