Protective Effects of a Jellyfish-Derived Thioredoxin Fused with Cell-Penetrating Peptide TAT-PTD on H2O2-Induced Oxidative Damage
- PMID: 37108504
- PMCID: PMC10138494
- DOI: 10.3390/ijms24087340
Protective Effects of a Jellyfish-Derived Thioredoxin Fused with Cell-Penetrating Peptide TAT-PTD on H2O2-Induced Oxidative Damage
Abstract
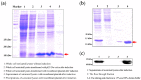
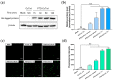
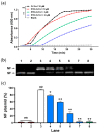
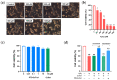
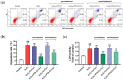
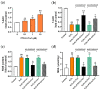
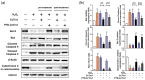
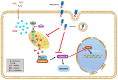
Thioredoxin (Trx) plays a critical role in maintaining redox balance in various cells and exhibits anti-oxidative, anti-apoptotic, and anti-inflammatory effects. However, whether exogenous Trx can inhibit intracellular oxidative damage has not been investigated. In previous study, we have identified a novel Trx from the jellyfish Cyanea capillata, named CcTrx1, and confirmed its antioxidant activities in vitro. Here, we obtained a recombinant protein, PTD-CcTrx1, which is a fusion of CcTrx1 and protein transduction domain (PTD) of HIV TAT protein. The transmembrane ability and antioxidant activities of PTD-CcTrx1, and its protective effects against H2O2-induced oxidative damage in HaCaT cells were also detected. Our results revealed that PTD-CcTrx1 exhibited specific transmembrane ability and antioxidant activities, and it could significantly attenuate the intracellular oxidative stress, inhibit H2O2-induced apoptosis, and protect HaCaT cells from oxidative damage. The present study provides critical evidence for application of PTD-CcTrx1 as a novel antioxidant to treat skin oxidative damage in the future.
Keywords: antioxidant; jellyfish; oxidative stress; protein transduction domain; thioredoxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Transduction of jellyfish superoxide dismutase mediated by TAT peptide ameliorates H2O2-induced oxidative stress in HaCaT cells.Sci Rep. 2024 Dec 28;14(1):31037. doi: 10.1038/s41598-024-82261-6. Sci Rep. 2024. PMID: 39730660 Free PMC article.
-
First report of a thioredoxin homologue in jellyfish: molecular cloning, expression and antioxidant activity of CcTrx1 from Cyanea capillata.PLoS One. 2014 May 13;9(5):e97509. doi: 10.1371/journal.pone.0097509. eCollection 2014. PLoS One. 2014. PMID: 24824597 Free PMC article.
-
Tat-Thioredoxin-like protein 1 attenuates ischemic brain injury by regulation of MAPKs and apoptosis signaling.BMB Rep. 2023 Apr;56(4):234-239. doi: 10.5483/BMBRep.2022-0184. BMB Rep. 2023. PMID: 36571143 Free PMC article.
-
The taming of the cell penetrating domain of the HIV Tat: myths and realities.J Control Release. 2007 Feb 12;117(2):148-62. doi: 10.1016/j.jconrel.2006.10.031. Epub 2006 Nov 17. J Control Release. 2007. PMID: 17196289 Free PMC article. Review.
-
Redox regulation of lung inflammation by thioredoxin.Antioxid Redox Signal. 2005 Jan-Feb;7(1-2):60-71. doi: 10.1089/ars.2005.7.60. Antioxid Redox Signal. 2005. PMID: 15650396 Review.
Cited by
-
Transduction of jellyfish superoxide dismutase mediated by TAT peptide ameliorates H2O2-induced oxidative stress in HaCaT cells.Sci Rep. 2024 Dec 28;14(1):31037. doi: 10.1038/s41598-024-82261-6. Sci Rep. 2024. PMID: 39730660 Free PMC article.
References
-
- Mohania D., Chandel S., Kumar P., Verma V., Digvijay K., Tripathi D., Choudhury K., Mitten S.K., Shah D. Ultraviolet radiations: Skin defense-damage mechanism. Adv. Exp. Med. Biol. 2017;996:71–87. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

