Structural Insights into M1 Muscarinic Acetylcholine Receptor Signaling Bias between Gαq and β-Arrestin through BRET Assays and Molecular Docking
- PMID: 37108518
- PMCID: PMC10138654
- DOI: 10.3390/ijms24087356
Structural Insights into M1 Muscarinic Acetylcholine Receptor Signaling Bias between Gαq and β-Arrestin through BRET Assays and Molecular Docking
Abstract
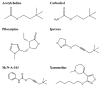
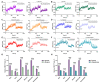
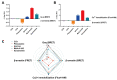
The selectivity of drugs for G protein-coupled receptor (GPCR) signaling pathways is crucial for their therapeutic efficacy. Different agonists can cause receptors to recruit effector proteins at varying levels, thus inducing different signaling responses, called signaling bias. Although several GPCR-biased drugs are currently being developed, only a limited number of biased ligands have been identified regarding their signaling bias for the M1 muscarinic acetylcholine receptor (M1mAChR), and the mechanism is not yet well understood. In this study, we utilized bioluminescence resonance energy transfer (BRET) assays to compare the efficacy of six agonists in inducing Gαq and β-arrestin2 binding to M1mAChR. Our findings reveal notable variations in agonist efficacy in the recruitment of Gαq and β-arrestin2. Pilocarpine preferentially promoted the recruitment of β-arrestin2 (∆∆RAi = -0.5), while McN-A-343 (∆∆RAi = 1.5), Xanomeline (∆∆RAi = 0.6), and Iperoxo (∆∆RAi = 0.3) exhibited a preference for the recruitment of Gαq. We also used commercial methods to verify the agonists and obtained consistent results. Molecular docking revealed that certain residues (e.g., Y404, located in TM7 of M1mAChR) could play crucial roles in Gαq signaling bias by interacting with McN-A-343, Xanomeline, and Iperoxo, whereas other residues (e.g., W378 and Y381, located in TM6) contributed to β-arrestin recruitment by interacting with Pilocarpine. The preference of activated M1mAChR for different effectors may be due to significant conformational changes induced by biased agonists. By characterizing bias towards Gαq and β-arrestin2 recruitment, our study provides insights into M1mAChR signaling bias.
Keywords: BRET; Gαq; M1 muscarinic acetylcholine receptor; molecular docking; signaling bias; β-arrestin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Functional selectivity profiling of the angiotensin II type 1 receptor using pathway-wide BRET signaling sensors.Sci Signal. 2018 Dec 4;11(559):eaat1631. doi: 10.1126/scisignal.aat1631. Sci Signal. 2018. PMID: 30514808
-
Bioluminescence Resonance Energy Transfer (BRET) to Detect the Interactions Between Kappa Opioid Receptor and Nonvisual Arrestins.Methods Mol Biol. 2021;2201:45-58. doi: 10.1007/978-1-0716-0884-5_5. Methods Mol Biol. 2021. PMID: 32975788
-
Unraveling allostery within the angiotensin II type 1 receptor for Gαq and β-arrestin coupling.Sci Signal. 2023 Aug 8;16(797):eadf2173. doi: 10.1126/scisignal.adf2173. Epub 2023 Aug 8. Sci Signal. 2023. PMID: 37552769 Free PMC article.
-
G protein-coupled receptor kinase type 2 and β-arrestin2: Key players in immune cell functions and inflammation.Cell Signal. 2022 Jul;95:110337. doi: 10.1016/j.cellsig.2022.110337. Epub 2022 Apr 21. Cell Signal. 2022. PMID: 35461901 Review.
-
Noncanonical interactions of G proteins and β-arrestins: from competitors to companions.FEBS J. 2021 Apr;288(8):2550-2561. doi: 10.1111/febs.15749. Epub 2021 Feb 22. FEBS J. 2021. PMID: 33539669 Review.
Cited by
-
Quantitative approaches for studying G protein-coupled receptor signalling and pharmacology.J Cell Sci. 2025 Jan 1;138(1):JCS263434. doi: 10.1242/jcs.263434. Epub 2025 Jan 15. J Cell Sci. 2025. PMID: 39810711 Free PMC article. Review.
-
The Activation of Muscarinic Acetylcholine Receptors Protects against Neuroinflammation in a Mouse Model through Attenuating Microglial Inflammation.Int J Mol Sci. 2024 Sep 27;25(19):10432. doi: 10.3390/ijms251910432. Int J Mol Sci. 2024. PMID: 39408758 Free PMC article.
References
-
- Werder R.B., Ullah M.A., Rahman M.M., Simpson J., Lynch J.P., Collinson N., Rittchen S., Rashid R.B., Sikder M.A.A., Handoko H.Y., et al. Targeting the P2Y(13) Receptor Suppresses IL-33 and HMGB1 Release and Ameliorates Experimental Asthma. Am. J. Respir. Crit. Care Med. 2022;205:300–312. doi: 10.1164/rccm.202009-3686OC. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

