Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells
- PMID: 37108554
- PMCID: PMC10138427
- DOI: 10.3390/ijms24087394
Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells
Abstract
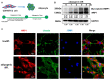
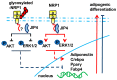
Neuropilin 1 (NRP1), a non-tyrosine kinase receptor for several ligands, is highly expressed in many kinds of mesenchymal stem cells (MSCs), but its function is poorly understood. In this study, we explored the roles of full-length NRP1 and glycosaminoglycan (GAG)-modifiable NRP1 in adipogenesis in C3H10T1/2 cells. The expression of full-length NRP1 and GAG-modifiable NRP1 increased during adipogenic differentiation in C3H10T1/2 cells. NRP1 knockdown repressed adipogenesis while decreasing the levels of Akt and ERK1/2 phosphorylation. Moreover, the scaffold protein JIP4 was involved in adipogenesis in C3H10T1/2 cells by interacting with NRP1. Furthermore, overexpression of non-GAG-modifiable NRP1 mutant (S612A) greatly promoted adipogenic differentiation, accompanied by upregulation of the phosphorylated Akt and ERK1/2. Taken together, these results indicate that NRP1 is a key regulator that promotes adipogenesis in C3H10T1/2 cells by interacting with JIP4 and activating the Akt and ERK1/2 pathway. Non-GAG-modifiable NRP1 mutant (S612A) accelerates the process of adipogenic differentiation, suggesting that GAG glycosylation is a negative post-translational modification of NRP1 in adipogenic differentiation.
Keywords: adipogenic differentiation; mesenchymal stem cells; neuropilin 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

