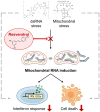
Resveratrol Attenuates the Mitochondrial RNA-Mediated Cellular Response to Immunogenic Stress
- PMID: 37108567
- PMCID: PMC10138523
- DOI: 10.3390/ijms24087403
Resveratrol Attenuates the Mitochondrial RNA-Mediated Cellular Response to Immunogenic Stress
Abstract
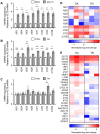
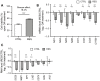
Human mitochondria contain a circular genome that encodes 13 subunits of the oxidative phosphorylation system. In addition to their role as powerhouses of the cells, mitochondria are also involved in innate immunity as the mitochondrial genome generates long double-stranded RNAs (dsRNAs) that can activate the dsRNA-sensing pattern recognition receptors. Recent evidence shows that these mitochondrial dsRNAs (mt-dsRNAs) are closely associated with the pathogenesis of human diseases that accompany inflammation and aberrant immune activation, such as Huntington's disease, osteoarthritis, and autoimmune Sjögren's syndrome. Yet, small chemicals that can protect cells from a mt-dsRNA-mediated immune response remain largely unexplored. Here, we investigate the potential of resveratrol (RES), a plant-derived polyphenol with antioxidant properties, on suppressing mt-dsRNA-mediated immune activation. We show that RES can revert the downstream response to immunogenic stressors that elevate mitochondrial RNA expressions, such as stimulation by exogenous dsRNAs or inhibition of ATP synthase. Through high-throughput sequencing, we find that RES can regulate mt-dsRNA expression, interferon response, and other cellular responses induced by these stressors. Notably, RES treatment fails to counter the effect of an endoplasmic reticulum stressor that does not affect the expression of mitochondrial RNAs. Overall, our study demonstrates the potential usage of RES to alleviate the mt-dsRNA-mediated immunogenic stress response.
Keywords: Sjögren’s syndrome; dsRNA stress; immunogenic stress; innate immunity; mitochondrial double-stranded RNAs; oligomycin A; resveratrol; tunicamycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

