Applied Compressive Strain Governs Hyaline-like Cartilage versus Fibrocartilage-like ECM Produced within Hydrogel Constructs
- PMID: 37108575
- PMCID: PMC10138702
- DOI: 10.3390/ijms24087410
Applied Compressive Strain Governs Hyaline-like Cartilage versus Fibrocartilage-like ECM Produced within Hydrogel Constructs
Abstract
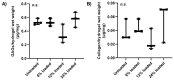
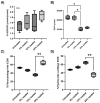
The goal of cartilage tissue engineering (CTE) is to regenerate new hyaline cartilage in joints and treat osteoarthritis (OA) using cell-impregnated hydrogel constructs. However, the production of an extracellular matrix (ECM) made of fibrocartilage is a potential outcome within hydrogel constructs when in vivo. Unfortunately, this fibrocartilage ECM has inferior biological and mechanical properties when compared to native hyaline cartilage. It was hypothesized that compressive forces stimulate fibrocartilage development by increasing production of collagen type 1 (Col1), an ECM protein found in fibrocartilage. To test the hypothesis, 3-dimensional (3D)-bioprinted hydrogel constructs were fabricated from alginate hydrogel impregnated with ATDC5 cells (a chondrogenic cell line). A bioreactor was used to simulate different in vivo joint movements by varying the magnitude of compressive strains and compare them with a control group that was not loaded. Chondrogenic differentiation of the cells in loaded and unloaded conditions was confirmed by deposition of cartilage specific molecules including glycosaminoglycans (GAGs) and collagen type 2 (Col2). By performing biochemical assays, the production of GAGs and total collagen was also confirmed, and their contents were quantitated in unloaded and loaded conditions. Furthermore, Col1 vs. Col2 depositions were assessed at different compressive strains, and hyaline-like cartilage vs. fibrocartilage-like ECM production was analyzed to investigate how applied compressive strain affects the type of cartilage formed. These assessments showed that fibrocartilage-like ECM production tended to reduce with increasing compressive strain, though its production peaked at a higher compressive strain. According to these results, the magnitude of applied compressive strain governs the production of hyaline-like cartilage vs. fibrocartilage-like ECM and a high compressive strain stimulates fibrocartilage-like ECM formation rather than hyaline cartilage, which needs to be addressed by CTE approaches.
Keywords: Col1; Col2; compressive force; fibrocartilage; hyaline cartilage; hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Reinforcement of Hydrogels with a 3D-Printed Polycaprolactone (PCL) Structure Enhances Cell Numbers and Cartilage ECM Production under Compression.J Funct Biomater. 2023 Jun 7;14(6):313. doi: 10.3390/jfb14060313. J Funct Biomater. 2023. PMID: 37367278 Free PMC article.
-
An in vitro and in vivo comparison of cartilage growth in chondrocyte-laden matrix metalloproteinase-sensitive poly(ethylene glycol) hydrogels with localized transforming growth factor β3.Acta Biomater. 2019 Jul 15;93:97-110. doi: 10.1016/j.actbio.2019.03.046. Epub 2019 Mar 23. Acta Biomater. 2019. PMID: 30914256 Free PMC article.
-
Cartilage Tissue Engineering Approaches Need to Assess Fibrocartilage When Hydrogel Constructs Are Mechanically Loaded.Front Bioeng Biotechnol. 2022 Jan 12;9:787538. doi: 10.3389/fbioe.2021.787538. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35096790 Free PMC article. Review.
-
Comparison study on hyaline cartilage versus fibrocartilage formation in a pig model by using 3D-bioprinted hydrogel and hybrid constructs.Biofabrication. 2024 Nov 5;17(1). doi: 10.1088/1758-5090/ad88a6. Biofabrication. 2024. PMID: 39423833
-
Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue.Adv Anat Embryol Cell Biol. 2005;178:1-71. Adv Anat Embryol Cell Biol. 2005. PMID: 16080262 Review.
Cited by
-
Development of a Nanoparticle System for Controlled Release in Bioprinted Respiratory Scaffolds.J Funct Biomater. 2024 Jan 12;15(1):20. doi: 10.3390/jfb15010020. J Funct Biomater. 2024. PMID: 38248687 Free PMC article.
-
Biomaterials / bioinks and extrusion bioprinting.Bioact Mater. 2023 Jun 27;28:511-536. doi: 10.1016/j.bioactmat.2023.06.006. eCollection 2023 Oct. Bioact Mater. 2023. PMID: 37435177 Free PMC article. Review.
-
Chondrogenic and Osteogenic In Vitro Differentiation Performance of Unsorted and Sorted CD34+, CD146+, and CD271+ Stem Cells Derived from Microfragmented Adipose Tissue of Patients with Knee Osteoarthritis.J Clin Med. 2025 Feb 11;14(4):1184. doi: 10.3390/jcm14041184. J Clin Med. 2025. PMID: 40004714 Free PMC article.
-
Reinforcement of Hydrogels with a 3D-Printed Polycaprolactone (PCL) Structure Enhances Cell Numbers and Cartilage ECM Production under Compression.J Funct Biomater. 2023 Jun 7;14(6):313. doi: 10.3390/jfb14060313. J Funct Biomater. 2023. PMID: 37367278 Free PMC article.
References
-
- Lee H.-S., Salter D.M. Osteoarthritis—Progress in Basic Research and Treatment. IntechOpen Limited; London, UK: 2015. Biomechanics of Cartilage and Osteoarthritis; pp. 41–52.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

