NTS, NTSR1 and ERs in the Pituitary-Gonadal Axis of Cycling and Postnatal Female Rats after BPA Treatment
- PMID: 37108581
- PMCID: PMC10138486
- DOI: 10.3390/ijms24087418
NTS, NTSR1 and ERs in the Pituitary-Gonadal Axis of Cycling and Postnatal Female Rats after BPA Treatment
Abstract
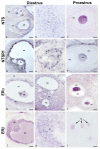
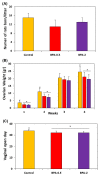
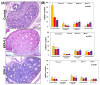
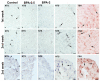
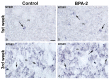
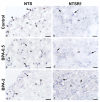
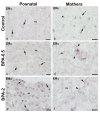
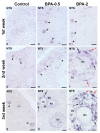
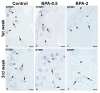
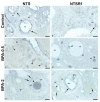
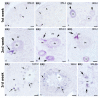
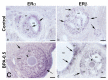
The neuropeptide neurotensin (NTS) is involved in regulating the reproductive axis and is expressed at each level of this axis (hypothalamus-pituitary-gonads). This dependence on estrogen levels has been widely demonstrated in the hypothalamus and pituitary. We focused on confirming the relationship of NTS with estrogens and the gonadal axis, using a particularly important environmental estrogenic molecule, bisphenol-A (BPA). Based on the experimental models or in vitro cell studies, it has been shown that BPA can negatively affect reproductive function. We studied for the first time the action of an exogenous estrogenic substance on the expression of NTS and estrogen receptors in the pituitary-gonadal axis during prolonged in vivo exposure. The exposure to BPA at 0.5 and 2 mg/kg body weight per day during gestation and lactation was monitored through indirect immunohistochemical procedures applied to the pituitary and ovary sections. Our results demonstrate that BPA induces alterations in the reproductive axis of the offspring, mainly after the first postnatal week. The rat pups exposed to BPA exhibited accelerated sexual maturation to puberty. There was no effect on the number of rats born per litter, although the fewer primordial follicles suggest a shorter fertile life.
Keywords: bisphenol-A; estrogen receptors; neurotensin; neurotensin receptor 1; ovaries; pituitary gland.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Delayed onset of puberty in male offspring from bisphenol A-treated dams is followed by the modulation of gene expression in the hypothalamic-pituitary-testis axis in adulthood.Reprod Fertil Dev. 2017 Nov;29(12):2496-2505. doi: 10.1071/RD17107. Reprod Fertil Dev. 2017. PMID: 28641706
-
Estrogen receptor α and β expressions in hypothalamus-pituitary-ovary axis in rats exposed lactationally to soy isoflavones and bisphenol A.Biomed Environ Sci. 2010 Oct;23(5):357-62. doi: 10.1016/S0895-3988(10)60076-1. Biomed Environ Sci. 2010. PMID: 21112483
-
Estrogen receptors are involved in xenoestrogen induction of growth hormone in the rat pituitary gland.J Reprod Dev. 2009 Apr;55(2):206-13. doi: 10.1262/jrd.20147. Epub 2009 Jan 15. J Reprod Dev. 2009. PMID: 19145065
-
Modulation of the hypothalamo-pituitary-gonadal axis and the pineal gland by neurokinin A, neuropeptide K and neuropeptide gamma.Peptides. 1999;20(2):285-99. doi: 10.1016/s0196-9781(98)00159-4. Peptides. 1999. PMID: 10422885 Review.
-
Bisphenol-A and Female Infertility: A Possible Role of Gene-Environment Interactions.Int J Environ Res Public Health. 2015 Sep 7;12(9):11101-16. doi: 10.3390/ijerph120911101. Int J Environ Res Public Health. 2015. PMID: 26371021 Free PMC article. Review.
Cited by
-
Impact of Bisphenol A and its alternatives on oocyte health: a scoping review.Hum Reprod Update. 2024 Dec 1;30(6):653-691. doi: 10.1093/humupd/dmae025. Hum Reprod Update. 2024. PMID: 39277428 Free PMC article.
-
Neurotensin Regulates Primate Ovulation Via Multiple Neurotensin Receptors.Endocrinology. 2025 Mar 24;166(5):bqaf041. doi: 10.1210/endocr/bqaf041. Endocrinology. 2025. PMID: 40037635 Free PMC article.
-
Neurotensin modulates the female reproductive system via extracellular signal-regulated kinase 1/2 signaling pathway: an in vivo and in silico study in mice.Mol Biol Rep. 2025 Jun 5;52(1):552. doi: 10.1007/s11033-025-10661-6. Mol Biol Rep. 2025. PMID: 40471330
References
-
- Alexander M.J., Dobner P.R., Miller M.A., Bullock B.P., Dorsa D.M., Leeman S.E. Estrogen induces neurotensin/neuromedin N messenger ribonucleic acid in a preoptic nucleus essential for the preovulatory surge of luteinizing hormone in the rat. Endocrinology. 1989;125:2111–2117. doi: 10.1210/endo-125-4-2111. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

