Development of an Inflammation-Triggered In Vitro "Leaky Gut" Model Using Caco-2/HT29-MTX-E12 Combined with Macrophage-like THP-1 Cells or Primary Human-Derived Macrophages
- PMID: 37108590
- PMCID: PMC10139037
- DOI: 10.3390/ijms24087427
Development of an Inflammation-Triggered In Vitro "Leaky Gut" Model Using Caco-2/HT29-MTX-E12 Combined with Macrophage-like THP-1 Cells or Primary Human-Derived Macrophages
Abstract
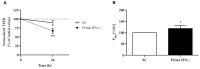
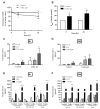
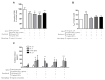
The "leaky gut" syndrome describes a damaged (leaky) intestinal mucosa and is considered a serious contributor to numerous chronic diseases. Chronic inflammatory bowel diseases (IBD) are particularly associated with the "leaky gut" syndrome, but also allergies, autoimmune diseases or neurological disorders. We developed a complex in vitro inflammation-triggered triple-culture model using 21-day-differentiated human intestinal Caco-2 epithelial cells and HT29-MTX-E12 mucus-producing goblet cells (90:10 ratio) in close contact with differentiated human macrophage-like THP-1 cells or primary monocyte-derived macrophages from human peripheral blood. Upon an inflammatory stimulus, the characteristics of a "leaky gut" became evident: a significant loss of intestinal cell integrity in terms of decreased transepithelial/transendothelial electrical resistance (TEER), as well as a loss of tight junction proteins. The cell permeability for FITC-dextran 4 kDa was then increased, and key pro-inflammatory cytokines, including TNF-alpha and IL-6, were substantially released. Whereas in the M1 macrophage-like THP-1 co-culture model, we could not detect the release of IL-23, which plays a crucial regulatory role in IBD, this cytokine was clearly detected when using primary human M1 macrophages instead. In conclusion, we provide an advanced human in vitro model that could be useful for screening and evaluating therapeutic drugs for IBD treatment, including potential IL-23 inhibitors.
Keywords: Caco-2; FITC-dextran 4 kDa (FD4); HT29-MTX-E12; TEER; THP-1; inflammation; inflammatory bowel disease (IBD); leaky gut; monocyte-derived macrophage; pro-inflammatory cytokines; triple-culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Investigating the Role of the NLRP3 Inflammasome Pathway in Acute Intestinal Inflammation: Use of THP-1 Knockout Cell Lines in an Advanced Triple Culture Model.Front Immunol. 2022 Jul 13;13:898039. doi: 10.3389/fimmu.2022.898039. eCollection 2022. Front Immunol. 2022. PMID: 35911682 Free PMC article.
-
Clinically relevant cell culture model of inflammatory bowel diseases for identification of new therapeutic approaches.Int J Pharm. 2025 Jan 25;669:125062. doi: 10.1016/j.ijpharm.2024.125062. Epub 2024 Dec 7. Int J Pharm. 2025. PMID: 39653295
-
Development and assessment of an intestinal tri-cellular model to investigate the pro/anti-inflammatory potential of digested foods.Front Immunol. 2025 Feb 5;16:1545261. doi: 10.3389/fimmu.2025.1545261. eCollection 2025. Front Immunol. 2025. PMID: 39975553 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease.Gastroenterology. 1997 May;112(5):1493-505. doi: 10.1016/s0016-5085(97)70030-1. Gastroenterology. 1997. PMID: 9136827 Review.
Cited by
-
Ultra-Processed Diets and Endocrine Disruption, Explanation of Missing Link in Rising Cancer Incidence Among Young Adults.Cancers (Basel). 2025 Jun 29;17(13):2196. doi: 10.3390/cancers17132196. Cancers (Basel). 2025. PMID: 40647494 Free PMC article. Review.
-
A Comparative Study on the Effects of Mesenchymal Stem Cells and Their Conditioned Medium on Caco-2 Cells as an In Vitro Model for Inflammatory Bowel Disease.Int J Cell Biol. 2024 Oct 21;2024:1022338. doi: 10.1155/2024/1022338. eCollection 2024. Int J Cell Biol. 2024. PMID: 39474054 Free PMC article.
-
Gastrointestinal tract, its pathophysiology and in-vitro models: A "quick" reference guide to translational studies.World J Gastroenterol. 2025 Jul 28;31(28):108297. doi: 10.3748/wjg.v31.i28.108297. World J Gastroenterol. 2025. PMID: 40741472 Free PMC article. Review.
-
Solving the Puzzle: Molecular Research in Inflammatory Bowel Diseases.Int J Mol Sci. 2023 Aug 29;24(17):13389. doi: 10.3390/ijms241713389. Int J Mol Sci. 2023. PMID: 37686195 Free PMC article.
-
Generation of Porcine and Rainbow Trout 3D Intestinal Models and Their Use to Investigate Astaxanthin Effects In Vitro.Int J Mol Sci. 2024 May 29;25(11):5966. doi: 10.3390/ijms25115966. Int J Mol Sci. 2024. PMID: 38892151 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

