Preclinical Evaluation of TB/FLU-04L-An Intranasal Influenza Vector-Based Boost Vaccine against Tuberculosis
- PMID: 37108602
- PMCID: PMC10138401
- DOI: 10.3390/ijms24087439
Preclinical Evaluation of TB/FLU-04L-An Intranasal Influenza Vector-Based Boost Vaccine against Tuberculosis
Abstract
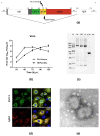
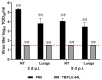
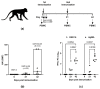
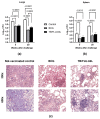
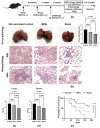
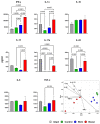
Tuberculosis is a major global threat to human health. Since the widely used BCG vaccine is poorly effective in adults, there is a demand for the development of a new type of boost tuberculosis vaccine. We designed a novel intranasal tuberculosis vaccine candidate, TB/FLU-04L, which is based on an attenuated influenza A virus vector encoding two mycobacterium antigens, Ag85A and ESAT-6. As tuberculosis is an airborne disease, the ability to induce mucosal immunity is one of the potential advantages of influenza vectors. Sequences of ESAT-6 and Ag85A antigens were inserted into the NS1 open reading frame of the influenza A virus to replace the deleted carboxyl part of the NS1 protein. The vector expressing chimeric NS1 protein appeared to be genetically stable and replication-deficient in mice and non-human primates. Intranasal immunization of C57BL/6 mice or cynomolgus macaques with the TB/FLU-04L vaccine candidate induced Mtb-specific Th1 immune response. Single TB/FLU-04L immunization in mice showed commensurate levels of protection in comparison to BCG and significantly increased the protective effect of BCG when applied in a "prime-boost" scheme. Our findings show that intranasal immunization with the TB/FLU-04L vaccine, which carries two mycobacterium antigens, is safe, and induces a protective immune response against virulent M. tuberculosis.
Keywords: Ag85A; ESAT-6; M. tuberculosis vaccine; influenza vector; mucosal immunization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures


Similar articles
-
Intranasal boosting with MVA encoding secreted mycobacterial proteins Ag85A and ESAT-6 generates strong pulmonary immune responses and protection against M. tuberculosis in mice given BCG as neonates.Vaccine. 2021 Mar 19;39(12):1780-1787. doi: 10.1016/j.vaccine.2021.01.071. Epub 2021 Feb 23. Vaccine. 2021. PMID: 33632562 Free PMC article.
-
A New Intranasal Influenza Vector-Based Vaccine TB/FLU-04L Against Tuberculosis: Preclinical Safety Studies.Drug Res (Stuttg). 2022 Jun;72(5):255-258. doi: 10.1055/a-1785-3936. Epub 2022 Mar 22. Drug Res (Stuttg). 2022. PMID: 35318622
-
Listeria-Vectored Multiantigenic Tuberculosis Vaccine Enhances Protective Immunity against Aerosol Challenge with Virulent Mycobacterium tuberculosis in BCG-Immunized C57BL/6 and BALB/c Mice.mBio. 2022 Jun 28;13(3):e0068722. doi: 10.1128/mbio.00687-22. Epub 2022 Jun 1. mBio. 2022. PMID: 35642945 Free PMC article.
-
Use of recombinant virus-vectored tuberculosis vaccines for respiratory mucosal immunization.Tuberculosis (Edinb). 2006 May-Jul;86(3-4):211-7. doi: 10.1016/j.tube.2006.01.017. Epub 2006 Feb 28. Tuberculosis (Edinb). 2006. PMID: 16504584 Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Vaccines against Tuberculosis: Where Are We Now?Vaccines (Basel). 2023 May 22;11(5):1013. doi: 10.3390/vaccines11051013. Vaccines (Basel). 2023. PMID: 37243117 Free PMC article. Review.
-
Bioinformatics Analysis and Immunogenicity Assessment of the Novel Multi-Stage DNA Vaccine W541 Against Mycobacterium Tuberculosis.Immun Inflamm Dis. 2024 Nov;12(11):e70074. doi: 10.1002/iid3.70074. Immun Inflamm Dis. 2024. PMID: 39588938 Free PMC article.
-
Safety and Protective Efficacy of a Candidate Vector-Based Vaccine for Bovine Tuberculosis.Vaccines (Basel). 2023 Jul 4;11(7):1199. doi: 10.3390/vaccines11071199. Vaccines (Basel). 2023. PMID: 37515015 Free PMC article.
-
Truncated NS1 Influenza A Virus Induces a Robust Antigen-Specific Tissue-Resident T-Cell Response and Promotes Inducible Bronchus-Associated Lymphoid Tissue Formation in Mice.Vaccines (Basel). 2025 Jan 10;13(1):58. doi: 10.3390/vaccines13010058. Vaccines (Basel). 2025. PMID: 39852837 Free PMC article.
-
The Use of Viral Vectors for Gene Therapy and Vaccination in Tuberculosis.Pharmaceuticals (Basel). 2023 Oct 16;16(10):1475. doi: 10.3390/ph16101475. Pharmaceuticals (Basel). 2023. PMID: 37895946 Free PMC article. Review.
References
-
- World Health Organization . Global Tuberculosis Report 2022. Volume 4. IGO; Geneva, Switzerland: 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

