Theaflavin Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo
- PMID: 37108608
- PMCID: PMC10138674
- DOI: 10.3390/ijms24087442
Theaflavin Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo
Abstract
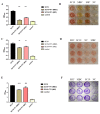
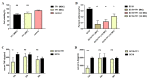
Streptococcus suis (S. suis) is one of the most important zoonotic pathogens that threaten the lives of pigs and humans. Even worse, the increasingly severe antimicrobial resistance in S. suis is becoming a global issue. Therefore, there is an urgent need to discover novel antibacterial alternatives for the treatment of S. suis infection. In this study, we investigated theaflavin (TF1), a benzoaphenone compound extracted from black tea, as a potential phytochemical compound against S. suis. TF1 at MIC showed significant inhibitory effects on S. suis growth, hemolytic activity, and biofilm formation, and caused damage to S. suis cells in vitro. TF1 had no cytotoxicity and decreased adherent activity of S. suis to the epithelial cell Nptr. Furthermore, TF1 not only improved the survival rate of S. suis-infected mice but also reduced the bacterial load and the production of IL-6 and TNF-α. A hemolysis test revealed the direct interaction between TF1 and Sly, while molecular docking showed TF1 had a good binding activity with the Glu198, Lys190, Asp111, and Ser374 of Sly. Moreover, virulence-related genes were downregulated in the TF1-treated group. Collectively, our findings suggested that TF1 can be used as a potential inhibitor for treating S. suis infection in view of its antibacterial and antihemolytic activity.
Keywords: Streptococcus suis; hemolytic activity; molecular docking; suilysin; theaflavin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Amentoflavone Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo.Appl Environ Microbiol. 2018 Nov 30;84(24):e01804-18. doi: 10.1128/AEM.01804-18. Print 2018 Dec 15. Appl Environ Microbiol. 2018. PMID: 30315078 Free PMC article.
-
Effective Antibacterial and Antihemolysin Activities of Ellipticine Hydrochloride against Streptococcus suis in a Mouse Model.Appl Environ Microbiol. 2021 Apr 27;87(10):e03165-20. doi: 10.1128/AEM.03165-20. Print 2021 Apr 27. Appl Environ Microbiol. 2021. PMID: 33674433 Free PMC article.
-
Ginkgetin in vitro and in vivo reduces Streptococcus suis virulence by inhibiting suilysin activity.J Appl Microbiol. 2019 Nov;127(5):1556-1563. doi: 10.1111/jam.14365. Epub 2019 Aug 15. J Appl Microbiol. 2019. PMID: 31260158
-
Biological activities of suilysin: role in Streptococcus suis pathogenesis.Future Microbiol. 2016 Jul;11:941-54. doi: 10.2217/fmb-2016-0028. Epub 2016 Jun 30. Future Microbiol. 2016. PMID: 27357518 Review.
-
Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part II - Pathogenesis.Ann Agric Environ Med. 2018 Mar 14;25(1):186-203. doi: 10.26444/aaem/85651. Epub 2018 Mar 2. Ann Agric Environ Med. 2018. PMID: 29575852 Review.
Cited by
-
Identification of SepF in Streptococcus suis involving cell division.BMC Microbiol. 2025 Mar 31;25(1):179. doi: 10.1186/s12866-025-03919-3. BMC Microbiol. 2025. PMID: 40165076 Free PMC article.
-
Study on the Effect of Phillyrin on Streptococcus suis In Vivo and In Vitro.Biomolecules. 2024 Dec 1;14(12):1542. doi: 10.3390/biom14121542. Biomolecules. 2024. PMID: 39766249 Free PMC article.
References
-
- Bag S., Mondal A., Majumder A., Banik A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2021;371:131098. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

