Effects of Nitric Oxide on the Activity of P2X and TRPV1 Receptors in Rat Meningeal Afferents of the Trigeminal Nerve
- PMID: 37108677
- PMCID: PMC10144808
- DOI: 10.3390/ijms24087519
Effects of Nitric Oxide on the Activity of P2X and TRPV1 Receptors in Rat Meningeal Afferents of the Trigeminal Nerve
Abstract
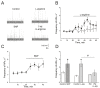
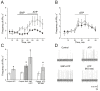
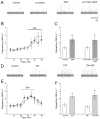
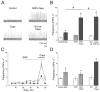
Nitric oxide is one of the endogenous molecules that play a key role in migraine. However, the interaction between NO and the main players in the nociceptive activity of the meningeal trigeminal afferents-TRPV1 and P2X3 receptors-remains unstudied. In the current project, the effects of acute and chronic NO administration on the activity of TRPV1 and P2X3 receptors in the peripheral afferents were studied using electrophysiological recording of action potentials of the trigeminal nerve in the rat hemiskull preparations. The data obtained indicate that exogenous and endogenous NO increased the activity of the trigeminal nerve independent on the inhibition of the TRPV1 and P2X3 receptors. The activity of the trigeminal nerve triggered by ATP changed neither in acute incubation in the NO donor-sodium nitroprusside (SNP) nor in the chronic nitroglycerine (NG)-induced migraine model. Moreover, the chronic NG administration did not increase in the number of degranulated mast cells in the rat meninges. At the same time, the capsaicin-induced activity of the trigeminal nerve was higher with chronic NO administration or after acute NO application, and these effects were prevented by N-ethylmaleimide. In conclusion, we suggested that NO positively modulates the activity of TRPV1 receptors by S-nitrosylation, which may contribute to the pro-nociceptive action of NO and underlie the sensitization of meningeal afferents in chronic migraine.
Keywords: P2X3 receptor; S-nitrosylation; TRPV1; mast cell; migraine; nitric oxide; nitroglycerine; trigeminal nerve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Implications of high homocysteine levels in migraine pain: An experimental study of the excitability of peripheral meningeal afferents in rats with hyperhomocysteinemia.Headache. 2024 May;64(5):533-546. doi: 10.1111/head.14710. Epub 2024 Apr 22. Headache. 2024. PMID: 38650105
-
Serotonergic mechanisms of trigeminal meningeal nociception: Implications for migraine pain.Neuropharmacology. 2017 Apr;116:160-173. doi: 10.1016/j.neuropharm.2016.12.024. Epub 2016 Dec 23. Neuropharmacology. 2017. PMID: 28025094
-
Migraine-relevant sex-dependent activation of mouse meningeal afferents by TRPM3 agonists.J Headache Pain. 2022 Jan 10;23(1):4. doi: 10.1186/s10194-021-01383-8. J Headache Pain. 2022. PMID: 35012445 Free PMC article.
-
Role of ATP in migraine mechanisms: focus on P2X3 receptors.J Headache Pain. 2023 Jan 3;24(1):1. doi: 10.1186/s10194-022-01535-4. J Headache Pain. 2023. PMID: 36597043 Free PMC article. Review.
-
Meningeal Mechanisms and the Migraine Connection.Annu Rev Neurosci. 2023 Jul 10;46:39-58. doi: 10.1146/annurev-neuro-080422-105509. Epub 2023 Mar 13. Annu Rev Neurosci. 2023. PMID: 36913712 Free PMC article. Review.
Cited by
-
Neurogasobiology of migraine: Carbon monoxide, hydrogen sulfide, and nitric oxide as emerging pathophysiological trinacrium relevant to nociception regulation.Open Med (Wars). 2025 May 17;20(1):20251201. doi: 10.1515/med-2025-1201. eCollection 2025. Open Med (Wars). 2025. PMID: 40391079 Free PMC article. Review.
-
Glycerol Trinitrate Acts Downstream of Calcitonin Gene-Related Peptide in Trigeminal Nociception-Evidence from Rodent Experiments with Anti-CGRP Antibody Fremanezumab.Cells. 2024 Mar 25;13(7):572. doi: 10.3390/cells13070572. Cells. 2024. PMID: 38607011 Free PMC article.
References
-
- Hermann A., Sitdikova G.F., Weiger T.M. Gasotransmitters: Physiology and Pathophysiology. Springer; Berlin, Heidelberg, Germany: 2012. pp. 1–204. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

