Acute Cardiovascular and Cardiorespiratory Effects of JWH-018 in Awake and Freely Moving Mice: Mechanism of Action and Possible Antidotal Interventions?
- PMID: 37108687
- PMCID: PMC10142259
- DOI: 10.3390/ijms24087515
Acute Cardiovascular and Cardiorespiratory Effects of JWH-018 in Awake and Freely Moving Mice: Mechanism of Action and Possible Antidotal Interventions?
Abstract
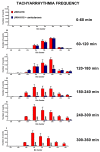
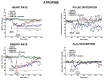
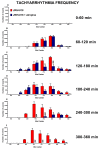
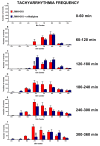
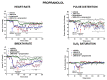
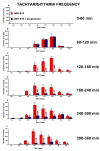
JWH-018 is the most known compound among synthetic cannabinoids (SCs) used for their psychoactive effects. SCs-based products are responsible for several intoxications in humans. Cardiac toxicity is among the main side effects observed in emergency departments: SCs intake induces harmful effects such as hypertension, tachycardia, chest pain, arrhythmias, myocardial infarction, breathing impairment, and dyspnea. This study aims to investigate how cardio-respiratory and vascular JWH-018 (6 mg/kg) responses can be modulated by antidotes already in clinical use. The tested antidotes are amiodarone (5 mg/kg), atropine (5 mg/kg), nifedipine (1 mg/kg), and propranolol (2 mg/kg). The detection of heart rate, breath rate, arterial oxygen saturation (SpO2), and pulse distention are provided by a non-invasive apparatus (Mouse Ox Plus) in awake and freely moving CD-1 male mice. Tachyarrhythmia events are also evaluated. Results show that while all tested antidotes reduce tachycardia and tachyarrhythmic events and improve breathing functions, only atropine completely reverts the heart rate and pulse distension. These data may suggest that cardiorespiratory mechanisms of JWH-018-induced tachyarrhythmia involve sympathetic, cholinergic, and ion channel modulation. Current findings also provide valuable impetus to identify potential antidotal intervention to support physicians in the treatment of intoxicated patients in emergency clinical settings.
Keywords: JWH-018; amiodarone; atropine; cardiovascular; nifedipine; propranolol; respiratory; synthetic cannabinoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Old and the New: Cardiovascular and Respiratory Alterations Induced by Acute JWH-018 Administration Compared to Δ9-THC-A Preclinical Study in Mice.Int J Mol Sci. 2023 Jan 13;24(2):1631. doi: 10.3390/ijms24021631. Int J Mol Sci. 2023. PMID: 36675144 Free PMC article.
-
Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings.Addiction. 2013 Mar;108(3):534-44. doi: 10.1111/j.1360-0443.2012.04078.x. Epub 2012 Nov 1. Addiction. 2013. PMID: 22971158
-
Novel halogenated synthetic cannabinoids impair sensorimotor functions in mice.Neurotoxicology. 2020 Jan;76:17-32. doi: 10.1016/j.neuro.2019.10.002. Epub 2019 Oct 11. Neurotoxicology. 2020. PMID: 31610187
-
5-HT2A receptors are involved in the pharmaco-toxicological effects of the synthetic cannabinoids JWH-018 and 5F-PB22: In vivo studies in mice.Eur J Pharmacol. 2024 May 15;971:176486. doi: 10.1016/j.ejphar.2024.176486. Epub 2024 Mar 6. Eur J Pharmacol. 2024. PMID: 38458413
-
Toxicity of Synthetic Cannabinoids in K2/Spice: A Systematic Review.Brain Sci. 2023 Jun 24;13(7):990. doi: 10.3390/brainsci13070990. Brain Sci. 2023. PMID: 37508922 Free PMC article. Review.
Cited by
-
Pharmaco-toxicological effects of the novel tryptamine hallucinogen 5-MeO-MiPT on motor, sensorimotor, physiological, and cardiorespiratory parameters in mice-from a human poisoning case to the preclinical evidence.Psychopharmacology (Berl). 2024 Mar;241(3):489-511. doi: 10.1007/s00213-024-06526-8. Epub 2024 Jan 12. Psychopharmacology (Berl). 2024. PMID: 38214743 Free PMC article.
References
-
- Pisarska A., Deluca P., Demetrovics Z., Moskalewicz J., ReDNet G. Novel psychoactive substances (NPS)-knowledge and expe riences of drug users from Hungary, Poland, the UK and the USA. Neuropsychopharmacol. Hung. 2019;21:152–163. - PubMed
-
- Kennedy J., Shanks K.G., Van Natta K., Prieto Conaway M.C., Wiseman J.M., Laughlin B., Kozak M. Rapid screening and identification of novel psychoactive substances using PaperSpray interfaced to high resolution mass spectrometry. Clin. Mass Spectrom. 2016;1:3–10. doi: 10.1016/j.clinms.2016.08.003. - DOI - PMC - PubMed
-
- Locatelli C.A., Lonati D., Petrolini V.M. New drugs of abuse and cardiovascular function. In: Govoni S., Politi P., Vanoli E., editors. Brain and Heart Dynamics. Springer; Cham, Switerland: 2020. pp. 1–27. - DOI
-
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) European Drug Report 2021: Trends and Developments. Publications Office of the European Union; Luxembourg: 2021.
-
- United Nations Office on Drugs and Crime (UNODC) Current NPS threats. [(accessed on 12 January 2023)]. Available online: https://www.unodc.org/documents/scientific/NPS_threats-IV.pdf.
MeSH terms
Substances
Grants and funding
- Effects of NPS: development of a multicentric research for the information enhancement of the Early Warning System/Anti-Drug Policies Department, Presidency of the Council of Ministers, Italy
- FAR 2021/University of Ferrara
- FAR 2022/University of Ferrara
- FIRB 2012 - Grant no. RBFR12LDOW/Italian Ministry of Education, University and Research
- Linea D1 grants/Catholic University of Rome
LinkOut - more resources
Full Text Sources

