GSK-3β Allosteric Inhibition: A Dead End or a New Pharmacological Frontier?
- PMID: 37108703
- PMCID: PMC10139115
- DOI: 10.3390/ijms24087541
GSK-3β Allosteric Inhibition: A Dead End or a New Pharmacological Frontier?
Abstract
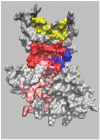
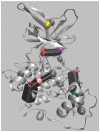
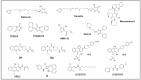
Most kinase inhibitors are designed to bind to highly homologous ATP-binding sites, which leads to promiscuity and possible off-target effects. Allostery is an alternative approach to pursuing selectivity. However, allostery is difficult to exploit due to the wide variety of underlying mechanisms and the potential involvement of long-range conformational effects that are difficult to pinpoint. GSK-3β is involved in several pathologies. This critical target has an ATP-binding site that is highly homologous with the orthosteric sites of other kinases. Unsurprisingly, there is also great similarity between the ATP-binding sites of GSK-3β and its isomer, which is not redundant and thus would benefit from selective inhibition. Allostery would also allow for a moderate and tunable inhibition, which is ideal for GSK-3β, because this target is involved in multiple pathways, some of which must be preserved. However, despite considerable research efforts, only one allosteric GSK-3β inhibitor has reached the clinic. Moreover, unlike other kinases, there are no X-ray structures of GSK-3β in complex with allosteric inhibitors in the PDB data bank. This review aims to summarize the state of the art in allosteric GSK-3β inhibitor investigations, highlighting the aspects that make this target challenging for an allosteric approach.
Keywords: Alzheimer’s disease; Pocketron; cancer; drug discovery; inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Amar S., Belmaker R.H., Agam G. The Possible Involvement of Glycogen Synthase Kinase-3 (GSK-3) in Diabetes, Cancer and Central Nervous System Diseases. Curr. Pharm. Des. 2011;17:2264–2277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

