Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels
- PMID: 37108709
- PMCID: PMC10141851
- DOI: 10.3390/ijms24087546
Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels
Abstract
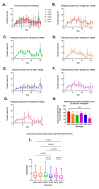
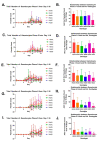
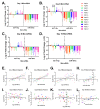
Malaria affects a significant portion of the global population, with 247 million cases in 2021, primarily in Africa. However, certain hemoglobinopathies, such as sickle cell trait (SCT), have been linked to lower mortality rates in malaria patients. Hemoglobin (Hb) mutations, including HbS and HbC, can cause sickle cell disease (SCD) when both alleles are inherited (HbSS and HbSC). In SCT, one allele is inherited and paired with a normal allele (HbAS, HbAC). The high prevalence of these alleles in Africa may be attributed to their protective effect against malaria. Biomarkers are crucial for SCD and malaria diagnosis and prognosis. Studies indicate that miRNAs, specifically miR-451a and let-7i-5p, are differentially expressed in HbSS and HbAS compared to controls. Our research examined the levels of exosomal miR-451a and let-7i-5p in red blood cells (RBCs) and infected red blood cells (iRBCs) from multiple sickle Hb genotypes and their impact on parasite growth. We assessed exosomal miR-451a and let-7i-5p levels in vitro in RBC and iRBC supernatants. Exosomal miRNAs exhibited distinct expression patterns in iRBCs from individuals with different sickle Hb genotypes. Additionally, we discovered a correlation between let-7i-5p levels and trophozoite count. Exosomal miR-451a and let-7i-5p could modulate SCD and malaria severity and serve as potential biomarkers for malaria vaccines and therapies.
Keywords: exosomes; extracellular microvesicles; malaria; microRNA; parasitemia; red blood cells; sickle cell disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
MicroRNAs miR-451a and Let-7i-5p Profiles in Circulating Exosomes Vary among Individuals with Different Sickle Hemoglobin Genotypes and Malaria.J Clin Med. 2022 Jan 19;11(3):500. doi: 10.3390/jcm11030500. J Clin Med. 2022. PMID: 35159951 Free PMC article.
-
MiR-451a and let-7i-5p loaded extracellular vesicles attenuate heme-induced inflammation in hiPSC-derived endothelial cells.Front Immunol. 2022 Dec 22;13:1082414. doi: 10.3389/fimmu.2022.1082414. eCollection 2022. Front Immunol. 2022. PMID: 36618355 Free PMC article.
-
Hemoglobin Genotypes Modulate Inflammatory Response to Plasmodium Infection.Front Immunol. 2020 Dec 23;11:593546. doi: 10.3389/fimmu.2020.593546. eCollection 2020. Front Immunol. 2020. PMID: 33424841 Free PMC article.
-
Review: the sickle hemoglobinopathies--genetic analyses of common phenocopies and new molecular approaches to treatment.Am J Med Sci. 1984 Nov;288(4):169-74. doi: 10.1097/00000441-198411000-00004. Am J Med Sci. 1984. PMID: 6208780 Review.
-
The diagnostic and prognostic value of exosomal microRNAs in lung cancer: a systematic review.Clin Transl Oncol. 2024 Aug;26(8):1921-1933. doi: 10.1007/s12094-024-03414-7. Epub 2024 Mar 15. Clin Transl Oncol. 2024. PMID: 38485857
Cited by
-
Modulation of Heme-Induced Inflammation Using MicroRNA-Loaded Liposomes: Implications for Hemolytic Disorders Such as Malaria and Sickle Cell Disease.Int J Mol Sci. 2023 Nov 29;24(23):16934. doi: 10.3390/ijms242316934. Int J Mol Sci. 2023. PMID: 38069257 Free PMC article.
-
Circulating biomarkers associated with pediatric sickle cell disease.Front Mol Biosci. 2024 Dec 19;11:1481441. doi: 10.3389/fmolb.2024.1481441. eCollection 2024. Front Mol Biosci. 2024. PMID: 39749215 Free PMC article.
-
Extracellular vesicles and endothelial dysfunction in infectious diseases.J Extracell Biol. 2024 Apr 12;3(4):e148. doi: 10.1002/jex2.148. eCollection 2024 Apr. J Extracell Biol. 2024. PMID: 38938849 Free PMC article. Review.
-
miR-215 Modulates Ubiquitination to Impair Inflammasome Activation and Autophagy During Salmonella Typhimurium Infection in Porcine Intestinal Cells.Animals (Basel). 2025 Feb 4;15(3):431. doi: 10.3390/ani15030431. Animals (Basel). 2025. PMID: 39943201 Free PMC article.
-
Encephalitis Unraveled: The Unlikely Encounter of Sickle Cell Disease and Cerebral Malaria in a Teenager.Diagnostics (Basel). 2025 Jun 10;15(12):1470. doi: 10.3390/diagnostics15121470. Diagnostics (Basel). 2025. PMID: 40564791 Free PMC article.
References
-
- WHO . World Health Organization Global Malaria Programme Report. WHO; Geneva, Switzerland: 2022. World malaria report 2022.
-
- Makani J., Komba A.N., Cox S.E., Oruo J., Mwamtemi K., Kitundu J., Magesa P., Rwezaula S., Meda E., Mgaya J., et al. Malaria in patients with sickle cell anemia: Burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115:215–220. doi: 10.1182/blood-2009-07-233528. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 5U54MD007602/MD/NIMHD NIH HHS/United States
- K01 TW010282/TW/FIC NIH HHS/United States
- 5R25TW009340/TW/FIC NIH HHS/United States
- TL1 TR002382/TR/NCATS NIH HHS/United States
- R01 NS091616/NS/NINDS NIH HHS/United States
- G12MD007602/MD/NIMHD NIH HHS/United States
- U54 MD007602/MD/NIMHD NIH HHS/United States
- G12 MD007602/MD/NIMHD NIH HHS/United States
- 1K01TW010282/TW/FIC NIH HHS/United States
- R25 TW009340/TW/FIC NIH HHS/United States
- 1R01NS091616/NS/NINDS NIH HHS/United States
- TL1TR002382/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

