How to Understand Personalized Medicine in Atopic Dermatitis Nowadays?
- PMID: 37108720
- PMCID: PMC10145758
- DOI: 10.3390/ijms24087557
How to Understand Personalized Medicine in Atopic Dermatitis Nowadays?
Abstract
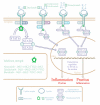
Atopic dermatitis (AD) is a heterogeneous disease in terms of its phenotypical, barrier, and immunological presentation. Emerging therapies are undoubtedly contributing to a new chapter in the treatment of AD, bringing an excellent possibility of individualization, and thereby creating a tailored approach. The two most promising substance groups are biological drugs (dupilumab, tralokinumab, lebrikizumab, nemolizumab) and Janus kinase inhibitors (JAKis) (baricitinib, upadacitinib, and abrocitinib). The vision that certain well-defined phenotypes and endotypes, as well as personal preferences, may guide the future treatment of AD is both tempting and appealing, but not yet reality. The accessibility of new drugs such as biologics and small molecules has opened up the discussion regarding personalized medicine, referring to the complex nature of AD as well as the experiences from clinical trials and real-world evidence. We have now reached the point of creating new strategies and AD treatment goals by increasing the amount of new information concerning the efficacy and safety of new drugs. This article has reviewed the novel treatment options for AD in the light of the heterogeneity of this disease and proposes a broader vision on the strategy of personalized treatment of AD.
Keywords: JAK inhibitors; atopic dermatitis; monoclonal antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wollenberg A., Kinberger M., Arents B., Aszodi N., Valle G.A., Barbarot S., Bieber T., Brough H., Pinton P.C., Christen-Zäch S., et al. European guideline (EuroGuiDerm) on atopic eczema: Part I—Systemic therapy. J. Eur. Acad. Dermatol. Venereol. 2022;36:1409–1431. doi: 10.1111/jdv.18345. - DOI - PubMed
-
- Wollenberg A., Kinberger M., Arents B., Aszodi N., Valle G.A., Barbarot S., Bieber T., Brough H., Pinton P.C., Christen-Zäch S., et al. European guideline (EuroGuiDerm) on atopic eczema—Part II: Non-systemic treatments and treatment recommendations for special AE patient populations. J. Eur. Acad. Dermatol. Venereol. 2022;36:1904–1926. doi: 10.1111/jdv.18429. - DOI - PubMed
-
- Nowicki R., Trzeciak M., Kaczmarski M., Wilkowska A., Czarnecka-Operacz M., Kowalewski C., Rudnicka L., Kulus M., Mastalerz-Migas A., Peregud-Pogorzelski J., et al. Atopic dermatitis. Interdisciplinary diagnostic and therapeutic recommendations of the Polish Dermatological Society, Polish Society of Allergology, Polish Pediatric Society and Polish Society of Family Medicine. Part I. Prophylaxis, topical treatment and phototherapy. Postep. Dermatol. Allergol. 2020;37:1–10. doi: 10.5114/ada.2020.93423. - DOI - PMC - PubMed
-
- Bosma A., Ascott A., Iskandar R., Farquhar K., Matthewman J., Langendam M., Mulick A., Abuabara K., Williams H., Spuls P., et al. Classifying atopic dermatitis: A systematic review of phenotypes and associated characteristics. J. Eur. Acad. Dermatol. Venereol. 2022;36:807–819. doi: 10.1111/jdv.18008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

