β-Tricalcium Phosphate-Modified Aerogel Containing PVA/Chitosan Hybrid Nanospun Scaffolds for Bone Regeneration
- PMID: 37108742
- PMCID: PMC10141662
- DOI: 10.3390/ijms24087562
β-Tricalcium Phosphate-Modified Aerogel Containing PVA/Chitosan Hybrid Nanospun Scaffolds for Bone Regeneration
Abstract
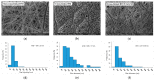
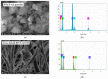
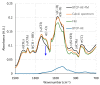
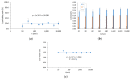
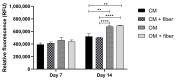
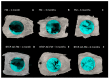
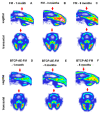
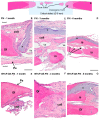
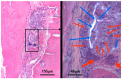
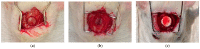
Electrospinning has recently been recognized as a potential method for use in biomedical applications such as nanofiber-based drug delivery or tissue engineering scaffolds. The present study aimed to demonstrate the electrospinning preparation and suitability of β-tricalcium phosphate-modified aerogel containing polyvinyl alcohol/chitosan fibrous meshes (BTCP-AE-FMs) for bone regeneration under in vitro and in vivo conditions. The mesh physicochemical properties included a 147 ± 50 nm fibrous structure, in aqueous media the contact angles were 64.1 ± 1.7°, and it released Ca, P, and Si. The viability of dental pulp stem cells on the BTCP-AE-FM was proven by an alamarBlue assay and with a scanning electron microscope. Critical-size calvarial defects in rats were performed as in vivo experiments to investigate the influence of meshes on bone regeneration. PET imaging using 18F-sodium fluoride standardized uptake values (SUVs) detected 7.40 ± 1.03 using polyvinyl alcohol/chitosan fibrous meshes (FMs) while 10.72 ± 1.11 with BTCP-AE-FMs after 6 months. New bone formations were confirmed by histological analysis. Despite a slight change in the morphology of the mesh because of cross-linking, the BTCP-AE-FM basically retained its fibrous, porous structure and hydrophilic and biocompatible character. Our experiments proved that hybrid nanospun scaffold composite mesh could be a new experimental bone substitute bioactive material in future medical practice.
Keywords: aerogel; electrospinning; electrospun meshes; tissue engineering; β-tricalcium phosphate.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Putra R.U., Basri H., Prakoso A.T., Chandra H., Ammarullah M.I., Akbar I., Syahrom A., Kamarul T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability. 2023;15:823. doi: 10.3390/su15010823. - DOI
-
- Qi Y., Wang C., Wang Q., Zhou F., Li T., Wang B., Su W., Shang D., Wu S. A Simple, Quick, and Cost-Effective Strategy to Fabricate Polycaprolactone/Silk Fibroin Nanofiber Yarns for Biotextile-Based Tissue Scaffold Application. Eur. Polym. J. 2023;186:111863. doi: 10.1016/j.eurpolymj.2023.111863. - DOI
-
- Li M., Qiu W., Wang Q., Li N., Liu L., Wang X., Yu J., Li X., Li F., Wu D. Nitric Oxide-Releasing Tryptophan-Based Poly(Ester Urea)s Electrospun Composite Nanofiber Mats with Antibacterial and Antibiofilm Activities for Infected Wound Healing. ACS Appl. Mater. Interfaces. 2022;14:15911–15926. doi: 10.1021/acsami.1c24131. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

