Comparative Physiological and Transcriptomic Mechanisms of Defoliation in Cotton in Response to Thidiazuron versus Ethephon
- PMID: 37108752
- PMCID: PMC10143250
- DOI: 10.3390/ijms24087590
Comparative Physiological and Transcriptomic Mechanisms of Defoliation in Cotton in Response to Thidiazuron versus Ethephon
Abstract
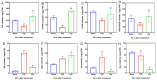
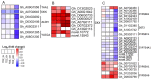
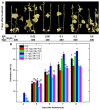
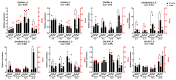
Thidiazuron (TDZ) is a widely used chemical defoliant in cotton and can stimulate the production of ethylene in leaves, which is believed to be the key factor in inducing leaf abscission. Ethephon (Eth) can also stimulate ethylene production in leaves, but it is less effective in promoting leaf shedding. In this study, the enzyme-linked immunosorbent assays (ELISA) and RNA-seq were used to determine specific changes at hormonal levels as well as transcriptomic mechanisms induced by TDZ compared with Eth. The TDZ significantly reduced the levels of auxin and cytokinin in cotton leaves, but no considerable changes were observed for Eth. In addition, TDZ specifically increased the levels of brassinosteroids and jasmonic acid in the leaves. A total of 13 764 differentially expressed genes that specifically responded to TDZ were identified by RNA-seq. The analysis of KEGG functional categories suggested that the synthesis, metabolism, and signal transduction of auxin, cytokinin, and brassinosteroid were all involved in the TDZ-induced abscission of cotton leaves. Eight auxin transport genes (GhPIN1-c_D, GhPIN3_D, GhPIN8_A, GhABCB19-b_A, GhABCB19-b_D, GhABCB2-b_D, GhLAX6_A, and GhLAX7_D) specifically responded to TDZ. The pro35S::GhPIN3a::YFP transgenic plants showed lower defoliation than the wild type treated with TDZ, and YFP fluorescence in leaves was almost extinguished after treatment with TDZ rather than Eth. This provides direct evidence that GhPIN3a is involved in the leaf abscission induced by TDZ. We found that 959 transcription factors (TFs) specifically responded to TDZ, and a co-expression network analysis (WGCNA) showed five hub TFs (GhNAC72, GhWRKY51, GhWRKY70, GhWRKY50, and GhHSF24) during chemical defoliation with TDZ. Our work sheds light on the molecular basis of TDZ-induced leaf abscission in cotton.
Keywords: auxin transport; brassinosteroid; cotton; cytokinin; leaf abscission; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Thidiazuron Promotes Leaf Abscission by Regulating the Crosstalk Complexities between Ethylene, Auxin, and Cytokinin in Cotton.Int J Mol Sci. 2022 Feb 28;23(5):2696. doi: 10.3390/ijms23052696. Int J Mol Sci. 2022. PMID: 35269837 Free PMC article.
-
Low Temperature Inhibits the Defoliation Efficiency of Thidiazuron in Cotton by Regulating Plant Hormone Synthesis and the Signaling Pathway.Int J Mol Sci. 2022 Nov 17;23(22):14208. doi: 10.3390/ijms232214208. Int J Mol Sci. 2022. PMID: 36430686 Free PMC article.
-
Thidiazuron combined with cyclanilide modulates hormone pathways and ROS systems in cotton, increasing defoliation at low temperatures.Front Plant Sci. 2024 Apr 3;15:1333816. doi: 10.3389/fpls.2024.1333816. eCollection 2024. Front Plant Sci. 2024. PMID: 38633458 Free PMC article.
-
The multipotent thidiazuron: A mechanistic overview of its roles in callogenesis and other plant cultures in vitro.Biotechnol Appl Biochem. 2022 Dec;69(6):2624-2640. doi: 10.1002/bab.2311. Epub 2022 Feb 8. Biotechnol Appl Biochem. 2022. PMID: 35048414 Review.
-
Is auxin enough? Cytokinins and margin patterning in simple leaves.Trends Plant Sci. 2023 Jan;28(1):54-73. doi: 10.1016/j.tplants.2022.08.019. Epub 2022 Sep 27. Trends Plant Sci. 2023. PMID: 36180378 Review.
Cited by
-
Comparative physiochemical and transcriptomic analysis reveals the influences of cross-pollination on ovary and fruit development in pummelo (Citrus maxima).Sci Rep. 2023 Nov 4;13(1):19081. doi: 10.1038/s41598-023-46058-3. Sci Rep. 2023. PMID: 37925539 Free PMC article.
-
Advances in cotton harvesting aids.Front Plant Sci. 2025 Apr 24;16:1570251. doi: 10.3389/fpls.2025.1570251. eCollection 2025. Front Plant Sci. 2025. PMID: 40343122 Free PMC article. Review.
-
Transcriptome differential expression analysis of defoliation of two different lemon varieties.PeerJ. 2024 Apr 26;12:e17218. doi: 10.7717/peerj.17218. eCollection 2024. PeerJ. 2024. PMID: 38685937 Free PMC article.
-
Advances in Molecular Plant Sciences.Int J Mol Sci. 2024 Jun 10;25(12):6408. doi: 10.3390/ijms25126408. Int J Mol Sci. 2024. PMID: 38928115 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

