Glutamine Deficiency Promotes Immune and Endothelial Cell Dysfunction in COVID-19
- PMID: 37108759
- PMCID: PMC10144995
- DOI: 10.3390/ijms24087593
Glutamine Deficiency Promotes Immune and Endothelial Cell Dysfunction in COVID-19
Abstract
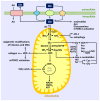
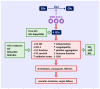
The coronavirus disease 2019 (COVID-19) pandemic has caused the death of almost 7 million people worldwide. While vaccinations and new antiviral drugs have greatly reduced the number of COVID-19 cases, there remains a need for additional therapeutic strategies to combat this deadly disease. Accumulating clinical data have discovered a deficiency of circulating glutamine in patients with COVID-19 that associates with disease severity. Glutamine is a semi-essential amino acid that is metabolized to a plethora of metabolites that serve as central modulators of immune and endothelial cell function. A majority of glutamine is metabolized to glutamate and ammonia by the mitochondrial enzyme glutaminase (GLS). Notably, GLS activity is upregulated in COVID-19, favoring the catabolism of glutamine. This disturbance in glutamine metabolism may provoke immune and endothelial cell dysfunction that contributes to the development of severe infection, inflammation, oxidative stress, vasospasm, and coagulopathy, which leads to vascular occlusion, multi-organ failure, and death. Strategies that restore the plasma concentration of glutamine, its metabolites, and/or its downstream effectors, in conjunction with antiviral drugs, represent a promising therapeutic approach that may restore immune and endothelial cell function and prevent the development of occlusive vascular disease in patients stricken with COVID-19.
Keywords: COVID-19; ammonia; coagulopathy; glutaminase; glutamine; heme oxygenase-1; immune and endothelial dysfunction; vascular disease.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Targeting Arginine in COVID-19-Induced Immunopathology and Vasculopathy.Metabolites. 2022 Mar 11;12(3):240. doi: 10.3390/metabo12030240. Metabolites. 2022. PMID: 35323682 Free PMC article. Review.
-
Metabolic fingerprinting reveals extensive consequences of GLS hyperactivity.Biochim Biophys Acta Gen Subj. 2020 Mar;1864(3):129484. doi: 10.1016/j.bbagen.2019.129484. Epub 2019 Nov 14. Biochim Biophys Acta Gen Subj. 2020. PMID: 31734463
-
Increased production of extracellular glutamate by the mitochondrial glutaminase following neuronal death.J Biol Chem. 1997 Apr 25;272(17):11276-82. doi: 10.1074/jbc.272.17.11276. J Biol Chem. 1997. PMID: 9111031
-
Glutaminase is essential for the growth of triple-negative breast cancer cells with a deregulated glutamine metabolism pathway and its suppression synergizes with mTOR inhibition.PLoS One. 2017 Sep 26;12(9):e0185092. doi: 10.1371/journal.pone.0185092. eCollection 2017. PLoS One. 2017. PMID: 28950000 Free PMC article.
-
The Glutamate-Glutamine Cycle in Epilepsy.Adv Neurobiol. 2016;13:351-400. doi: 10.1007/978-3-319-45096-4_14. Adv Neurobiol. 2016. PMID: 27885637 Review.
Cited by
-
Underlying Piezo2 Channelopathy-Induced Neural Switch of COVID-19 Infection.Cells. 2025 Jul 31;14(15):1182. doi: 10.3390/cells14151182. Cells. 2025. PMID: 40801614 Free PMC article. Review.
-
Glutamine Protects against Mouse Abdominal Aortic Aneurysm through Modulating VSMC Apoptosis and M1 Macrophage Activation.Int J Med Sci. 2024 May 19;21(8):1414-1427. doi: 10.7150/ijms.96395. eCollection 2024. Int J Med Sci. 2024. PMID: 38903916 Free PMC article.
-
Dietary Acid Load Correlates with Serum Amino Acid Concentrations after a Four-Week Intervention with Vegan vs. Meat-Rich Diets: A Secondary Data Analysis.Nutrients. 2023 Jun 28;15(13):2942. doi: 10.3390/nu15132942. Nutrients. 2023. PMID: 37447267 Free PMC article.
-
Identification of glutamine as a potential therapeutic target in dry eye disease.Signal Transduct Target Ther. 2025 Jan 22;10(1):27. doi: 10.1038/s41392-024-02119-1. Signal Transduct Target Ther. 2025. PMID: 39837870 Free PMC article.
-
Association between malnutrition and post-acute COVID-19 sequelae: A retrospective cohort study.JPEN J Parenter Enteral Nutr. 2024 Nov;48(8):906-916. doi: 10.1002/jpen.2662. Epub 2024 Jun 25. JPEN J Parenter Enteral Nutr. 2024. PMID: 38924100 Free PMC article.
References
-
- World Health Organization WHO Coronavirus Disease (COVID) Dashboard. 2023. [(accessed on 28 March 2023)]. Available online: http:/covid19.who.int.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

