A Decellularized Uterine Endometrial Scaffold Enhances Regeneration of the Endometrium in Rats
- PMID: 37108764
- PMCID: PMC10145056
- DOI: 10.3390/ijms24087605
A Decellularized Uterine Endometrial Scaffold Enhances Regeneration of the Endometrium in Rats
Abstract
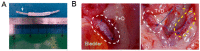
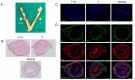
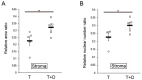
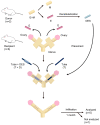
Partial or whole regeneration of the uterine endometrium using extracellular matrix (ECM)-based scaffolds is a therapeutic strategy for uterine infertility due to functional and/or structural endometrial defects. Here, we examined whether the entire endometrium can be regenerated circumferentially using an acellular ECM scaffold (decellularized endometrial scaffold, DES) prepared from rat endometrium. We placed a silicone tube alone to prevent adhesions or a DES loaded with a silicone tube into a recipient uterus in which the endometrium had been surgically removed circumferentially. Histological and immunofluorescent analyses of the uteri one month after tube placement revealed more abundant regenerated endometrial stroma in the uterine horns treated with tube-loaded DES compared to those treated with a tube alone. Luminal and glandular epithelia, however, were not fully recapitulated. These results suggest that DES can enhance the regeneration of endometrial stroma but additional intervention(s) are needed to induce epithelization. Furthermore, the prevention of adhesions alone allowed the endometrial stroma to regenerate circumferentially even without a DES, but to a lesser degree than that with a DES. The use of a DES together with the prevention of adhesions may be beneficial for efficient endometrial regeneration in the uterus that is largely deficient of endometrium.
Keywords: ECM (extracellular matrix); endometrium; regenerative medicine; scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Johannesson L., Richards E., Reddy V., Walter J., Olthoff K., Quintini C., Tzakis A., Latif N., Porrett P., O’Neill K., et al. The First 5 Years of Uterus Transplant in the US: A Report From the United States Uterus Transplant Consortium. JAMA Surg. 2022;157:790–797. doi: 10.1001/jamasurg.2022.2612. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

