Enzymatic Synthesis of a Novel Coumarin Aminophosphonates: Antibacterial Effects and Oxidative Stress Modulation on Selected E. coli Strains
- PMID: 37108774
- PMCID: PMC10146307
- DOI: 10.3390/ijms24087609
Enzymatic Synthesis of a Novel Coumarin Aminophosphonates: Antibacterial Effects and Oxidative Stress Modulation on Selected E. coli Strains
Abstract
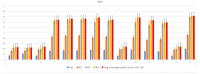
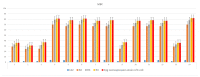
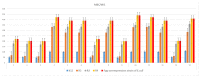
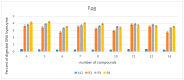
The objective of the present study was to evaluate the synergistic effect of two important pharmacophores, coumarin and α-amino dimethyl phosphonate moieties, on antimicrobial activity toward selected LPS-varied E. coli strains. Studied antimicrobial agents were prepared via a Kabachnik-Fields reaction promoted by lipases. The products were provided with an excellent yield (up to 92%) under mild, solvent- and metal-free conditions. A preliminary exploration of coumarin α-amino dimethyl phosphonate analogs as novel antimicrobial agents was carried out to determine the basic features of the structure responsible for the observed biological activity. The structure-activity relationship revealed that an inhibitory activity of the synthesized compounds is strongly related to the type of the substituents located in the phenyl ring. The collected data demonstrated that coumarin-based α-aminophosphonates can be potential antimicrobial drug candidates, which is particularly crucial due to the constantly increasing resistance of bacteria to commonly used antibiotics.
Keywords: E. coli cells; Kabachnik-Fields reaction; MIC; aminophosphonates; antimicrobial activity; coumarins; enzymatic catalytic promiscuity; lipases; multicomponent reaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Evaluation of Antibacterial Activity against Nosocomial Pathogens of an Enzymatically Derived α-Aminophosphonates Possessing Coumarin Scaffold.Int J Mol Sci. 2023 Oct 4;24(19):14886. doi: 10.3390/ijms241914886. Int J Mol Sci. 2023. PMID: 37834334 Free PMC article.
-
Synthesis and potent antimicrobial activity of novel coumarylthiazole α-aminophosphonates derivatives.Mol Divers. 2022 Apr;26(2):1161-1174. doi: 10.1007/s11030-021-10242-2. Epub 2021 Jun 12. Mol Divers. 2022. PMID: 34117993
-
Promiscuous Lipase-Catalyzed Knoevenagel-Phospha-Michael Reaction for the Synthesis of Antimicrobial β-Phosphono Malonates.Int J Mol Sci. 2022 Aug 8;23(15):8819. doi: 10.3390/ijms23158819. Int J Mol Sci. 2022. PMID: 35955950 Free PMC article.
-
A Review on Anti-urease Potential of Coumarins.Curr Drug Targets. 2021;22(17):1926-1943. doi: 10.2174/1389450122666210222091412. Curr Drug Targets. 2021. PMID: 33618646 Review.
-
Coumarin-containing hybrids and their antibacterial activities.Arch Pharm (Weinheim). 2020 Jun;353(6):e1900380. doi: 10.1002/ardp.201900380. Epub 2020 Apr 6. Arch Pharm (Weinheim). 2020. PMID: 32253782 Review.
Cited by
-
Evaluation of Antibacterial Activity against Nosocomial Pathogens of an Enzymatically Derived α-Aminophosphonates Possessing Coumarin Scaffold.Int J Mol Sci. 2023 Oct 4;24(19):14886. doi: 10.3390/ijms241914886. Int J Mol Sci. 2023. PMID: 37834334 Free PMC article.
-
Coumarin: A natural solution for alleviating inflammatory disorders.Curr Res Pharmacol Drug Discov. 2024 Sep 25;7:100202. doi: 10.1016/j.crphar.2024.100202. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 39398983 Free PMC article. Review.
-
Antimicrobial activity of Ruta angustifolia L. Pers against periodontal pathogen: Porphyromonas gingivalis.PeerJ. 2024 Dec 18;12:e18751. doi: 10.7717/peerj.18751. eCollection 2024. PeerJ. 2024. PMID: 39713137 Free PMC article.
-
Novel coumarin linked pyrazoles, thiazoles, and thiadiazoles: synthetic strategies and in vitro antimicrobial investigation.Future Med Chem. 2025 Jan;17(2):183-193. doi: 10.1080/17568919.2024.2444867. Epub 2024 Dec 26. Future Med Chem. 2025. PMID: 39723730
-
Syntheses, reactivity, and biological applications of coumarins.Front Chem. 2024 Feb 19;12:1362992. doi: 10.3389/fchem.2024.1362992. eCollection 2024. Front Chem. 2024. PMID: 38440776 Free PMC article. Review.
References
-
- IACG . Interagency Coordination Group on Antimicrobial Resistance (2019) No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. IACG; Hyderabad, India: 2019. p. 28.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

