Specific Activation of T Cells by an ACE2-Based CAR-Like Receptor upon Recognition of SARS-CoV-2 Spike Protein
- PMID: 37108807
- PMCID: PMC10145580
- DOI: 10.3390/ijms24087641
Specific Activation of T Cells by an ACE2-Based CAR-Like Receptor upon Recognition of SARS-CoV-2 Spike Protein
Abstract
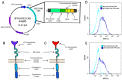
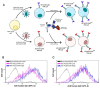
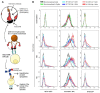
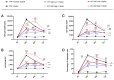
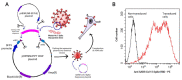
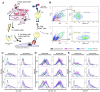
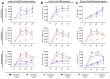
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the causative agent of the Coronavirus Disease 2019 (COVID-19) pandemic, which is still a health issue worldwide mostly due to a high rate of contagiousness conferred by the high-affinity binding between cell viral receptors, Angiotensin-Converting Enzyme 2 (ACE2) and SARS-CoV-2 Spike protein. Therapies have been developed that rely on the use of antibodies or the induction of their production (vaccination), but despite vaccination being still largely protective, the efficacy of antibody-based therapies wanes with the advent of new viral variants. Chimeric Antigen Receptor (CAR) therapy has shown promise for tumors and has also been proposed for COVID-19 treatment, but as recognition of CARs still relies on antibody-derived sequences, they will still be hampered by the high evasion capacity of the virus. In this manuscript, we show the results from CAR-like constructs with a recognition domain based on the ACE2 viral receptor, whose ability to bind the virus will not wane, as Spike/ACE2 interaction is pivotal for viral entry. Moreover, we have developed a CAR construct based on an affinity-optimized ACE2 and showed that both wild-type and affinity-optimized ACE2 CARs drive activation of a T cell line in response to SARS-CoV-2 Spike protein expressed on a pulmonary cell line. Our work sets the stage for the development of CAR-like constructs against infectious agents that would not be affected by viral escape mutations and could be developed as soon as the receptor is identified.
Keywords: CAR-like receptor; COVID-19; Chimeric Antigen Receptors (CAR); SARS-CoV-2; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19.Front Immunol. 2021 Dec 22;12:732690. doi: 10.3389/fimmu.2021.732690. eCollection 2021. Front Immunol. 2021. PMID: 35003058 Free PMC article. Review.
-
Characterization of SARS-CoV-2 Variants B.1.617.1 (Kappa), B.1.617.2 (Delta), and B.1.618 by Cell Entry and Immune Evasion.mBio. 2022 Apr 26;13(2):e0009922. doi: 10.1128/mbio.00099-22. Epub 2022 Mar 10. mBio. 2022. PMID: 35266815 Free PMC article.
-
S309-CAR-NK cells bind the Omicron variants in vitro and reduce SARS-CoV-2 viral loads in humanized ACE2-NSG mice.J Virol. 2024 Jun 13;98(6):e0003824. doi: 10.1128/jvi.00038-24. Epub 2024 May 20. J Virol. 2024. PMID: 38767356 Free PMC article.
-
Competitive SARS-CoV-2 Serology Reveals Most Antibodies Targeting the Spike Receptor-Binding Domain Compete for ACE2 Binding.mSphere. 2020 Sep 16;5(5):e00802-20. doi: 10.1128/mSphere.00802-20. mSphere. 2020. PMID: 32938700 Free PMC article.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
Cited by
-
Next-generation treatments: Immunotherapy and advanced therapies for COVID-19.Heliyon. 2024 Feb 19;10(5):e26423. doi: 10.1016/j.heliyon.2024.e26423. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38434363 Free PMC article. Review.
-
Advanced Therapy Medicinal Products as Potential Therapeutic Strategy against COVID-19 and Immune-Related Disorders.Int J Mol Sci. 2024 Mar 6;25(5):3079. doi: 10.3390/ijms25053079. Int J Mol Sci. 2024. PMID: 38474324 Free PMC article.
-
In vivo CAR engineering for immunotherapy.Nat Rev Immunol. 2025 May 16. doi: 10.1038/s41577-025-01174-1. Online ahead of print. Nat Rev Immunol. 2025. PMID: 40379910 Review.
-
The subventricular zone neurogenic niche provides adult born functional neurons to repair cortical brain injuries in response to diterpenoid therapy.Stem Cell Res Ther. 2025 Jan 5;16(1):1. doi: 10.1186/s13287-024-04105-4. Stem Cell Res Ther. 2025. PMID: 39757190 Free PMC article.
-
Discordant CAR-T cell signaling: implications of divergence from physiological T cell activation.J Transl Med. 2025 Jul 25;23(1):834. doi: 10.1186/s12967-025-06857-w. J Transl Med. 2025. PMID: 40713606 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

