Leveraging Exosomes as the Next-Generation Bio-Shuttles: The Next Biggest Approach against Th17 Cell Catastrophe
- PMID: 37108809
- PMCID: PMC10142210
- DOI: 10.3390/ijms24087647
Leveraging Exosomes as the Next-Generation Bio-Shuttles: The Next Biggest Approach against Th17 Cell Catastrophe
Abstract
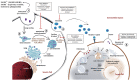
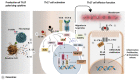
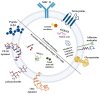
In recent years, the launch of clinical-grade exosomes is rising expeditiously, as they represent a new powerful approach for the delivery of advanced therapies and for diagnostic purposes for various diseases. Exosomes are membrane-bound extracellular vesicles that can act as biological messengers between cells, in the context of health and disease. In comparison to several lab-based drug carriers, exosome exhibits high stability, accommodates diverse cargo loads, elicits low immunogenicity and toxicity, and therefore manifests tremendous perspectives in the development of therapeutics. The efforts made to spur exosomes in drugging the untreatable targets are encouraging. Currently, T helper (Th) 17 cells are considered the most prominent factor in the establishment of autoimmunity and several genetic disorders. Current reports have indicated the importance of targeting the development of Th17 cells and the secretion of its paracrine molecule, interleukin (IL)-17. However, the present-day targeted approaches exhibit drawbacks, such as high cost of production, rapid transformation, poor bioavailability, and importantly, causing opportunistic infections that ultimately hamper their clinical applications. To overcome this hurdle, the potential use of exosomes as vectors seem to be a promising approach for Th17 cell-targeted therapies. With this standpoint, this review discusses this new concept by providing a snapshot of exosome biogenesis, summarizes the current clinical trials of exosomes in several diseases, analyzes the prospect of exosomes as an established drug carrier and delineates the present challenges, with an emphasis on their practical applications in targeting Th17 cells in diseases. We further decode the possible future scope of exosome bioengineering for targeted drug delivery against Th17 cells and its catastrophe.
Keywords: Th17 cell; drug delivery vector; exosome engineering; packaging therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Exosome engineering in cell therapy and drug delivery.Inflammopharmacology. 2023 Feb;31(1):145-169. doi: 10.1007/s10787-022-01115-7. Epub 2023 Jan 7. Inflammopharmacology. 2023. PMID: 36609717 Free PMC article. Review.
-
Engineering Approaches for Exosome Cargo Loading and Targeted Delivery: Biological versus Chemical Perspectives.ACS Biomater Sci Eng. 2024 Oct 14;10(10):5960-5976. doi: 10.1021/acsbiomaterials.4c00856. Epub 2024 Jun 28. ACS Biomater Sci Eng. 2024. PMID: 38940421 Review.
-
Exosome engineering for efficient and targeted drug delivery: Current status and future perspective.J Physiol. 2023 Nov;601(22):4853-4872. doi: 10.1113/JP282799. Epub 2022 Jun 2. J Physiol. 2023. PMID: 35570717 Review.
-
Challenges in the development and establishment of exosome-based drug delivery systems.J Control Release. 2021 Jan 10;329:894-906. doi: 10.1016/j.jconrel.2020.10.020. Epub 2020 Oct 12. J Control Release. 2021. PMID: 33058934 Review.
-
Exosomes: Cell-Derived Nanoplatforms for the Delivery of Cancer Therapeutics.Int J Mol Sci. 2020 Dec 22;22(1):14. doi: 10.3390/ijms22010014. Int J Mol Sci. 2020. PMID: 33374978 Free PMC article. Review.
Cited by
-
Extracellular Vesicles: Biology and Therapeutic Applications.Int J Mol Sci. 2024 Dec 4;25(23):13034. doi: 10.3390/ijms252313034. Int J Mol Sci. 2024. PMID: 39684744 Free PMC article.
-
Exosomal non-coding RNAs: gatekeepers of inflammation in autoimmune disease.J Inflamm (Lond). 2025 May 14;22(1):18. doi: 10.1186/s12950-025-00443-z. J Inflamm (Lond). 2025. PMID: 40369549 Free PMC article. Review.
-
Exosomes in inflammation and cancer: from bench to bedside applications.Mol Biomed. 2025 Jun 10;6(1):41. doi: 10.1186/s43556-025-00280-9. Mol Biomed. 2025. PMID: 40490663 Free PMC article. Review.
-
Exosomal delivery of non-coding RNAs: a pathway to apoptosis regulation in lung cancer.Med Oncol. 2025 Jul 1;42(8):307. doi: 10.1007/s12032-025-02852-9. Med Oncol. 2025. PMID: 40591081 Review.
References
-
- Lin A., Giuliano C.J., Palladino A., John K.M., Abramowicz C., Yuan M.L., Sausville E.L., Lukow D.A., Liu L., Chait A.R., et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 2019;11:eaaw8412. doi: 10.1126/scitranslmed.aaw8412. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

