Pneumocystis Exacerbates Inflammation and Mucus Hypersecretion in a Murine, Elastase-Induced-COPD Model
- PMID: 37108906
- PMCID: PMC10142929
- DOI: 10.3390/jof9040452
Pneumocystis Exacerbates Inflammation and Mucus Hypersecretion in a Murine, Elastase-Induced-COPD Model
Abstract
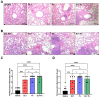
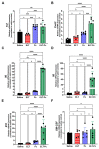
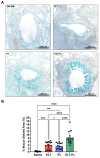
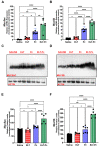
Inflammation and mucus hypersecretion are frequent pathology features of chronic respiratory diseases such as asthma and COPD. Selected bacteria, viruses and fungi may synergize as co-factors in aggravating disease by activating pathways that are able to induce airway pathology. Pneumocystis infection induces inflammation and mucus hypersecretion in immune competent and compromised humans and animals. This fungus is a frequent colonizer in patients with COPD. Therefore, it becomes essential to identify whether it has a role in aggravating COPD severity. This work used an elastase-induced COPD model to evaluate the role of Pneumocystis in the exacerbation of pathology, including COPD-like lung lesions, inflammation and mucus hypersecretion. Animals infected with Pneumocystis developed increased histology features of COPD, inflammatory cuffs around airways and lung vasculature plus mucus hypersecretion. Pneumocystis induced a synergic increment in levels of inflammation markers (Cxcl2, IL6, IL8 and IL10) and mucins (Muc5ac/Muc5b). Levels of STAT6-dependent transcription factors Gata3, FoxA3 and Spdef were also synergically increased in Pneumocystis infected animals and elastase-induced COPD, while the levels of the mucous cell-hyperplasia transcription factor FoxA2 were decreased compared to the other groups. Results document that Pneumocystis is a co-factor for disease severity in this elastase-induced-COPD model and highlight the relevance of STAT6 pathway in Pneumocystis pathogenesis.
Keywords: COPD; Muc5ac; Muc5b; Pneumocystis; hypersecretion; inflammation; mucins; mucus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- McDonough J.E., Yuan R., Suzuki M., Seyednejad N., Elliott W.M., Sanchez P.G., Wright A.C., Gefter W.B., Litzky L., Coxson H.O., et al. Small-Airway Obstruction and Emphysema in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. - DOI - PMC - PubMed
-
- Retamales I., Elliott W.M., Meshi B., Coxson H.O., Pare P.D., Sciurba F.C., Rogers R.M., Hayashi S., Hogg J.C. Amplification of Inflammation in Emphysema and Its Association with Latent Adenoviral Infection. Am. J. Respir. Crit. Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

