Amesia hispanica sp. nov., Producer of the Antifungal Class of Antibiotics Dactylfungins
- PMID: 37108917
- PMCID: PMC10141101
- DOI: 10.3390/jof9040463
Amesia hispanica sp. nov., Producer of the Antifungal Class of Antibiotics Dactylfungins
Abstract
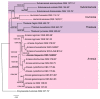
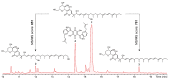
During a study of the diversity of soilborne fungi from Spain, a strain belonging to the family Chaetomiaceae (Sordariales) was isolated. The multigene phylogenetic inference using five DNA loci showed that this strain represents an undescribed species of the genus Amesia, herein introduced as A. hispanica sp. nov. Investigation of its secondary metabolome led to the isolation of two new derivatives (2 and 3) of the known antifungal antibiotic dactylfungin A (1), together with the known compound cochliodinol (4). The planar structures of 1-4 were determined by ultrahigh performance liquid chromatography coupled with diode array detection and ion mobility tandem mass spectrometry (UHPLC-DAD-IM-MS/MS) and extensive 1D and 2D nuclear magnetic resonance (NMR) spectroscopy after isolation by HPLC. All isolated secondary metabolites were tested for their antimicrobial and cytotoxic activities. Dactylfungin A (1) showed selective and strong antifungal activity against some of the tested human pathogens (Aspergillus fumigatus and Cryptococcus neoformans). The additional hydroxyl group in 2 resulted in the loss of activity against C. neoformans but still retained the inhibition of As. fumigatus in a lower concentration than that of the respective control, without showing any cytotoxic effects. In contrast, 25″-dehydroxy-dactylfungin A (3) exhibited improved activity against yeasts (Schizosaccharomyces pombe and Rhodotorula glutinis) than 1 and 2, but resulted in the appearance of slight cytotoxicity. The present study exemplifies how even in a well-studied taxonomic group such as the Chaetomiaceae, the investigation of novel taxa still brings chemistry novelty, as demonstrated in this first report of this antibiotic class for chaetomiaceous and sordarialean taxa.
Keywords: Chaetomiaceae; Sordariales; antifungals; fungal secondary metabolites; metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

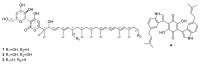
References
-
- Hyde K.D., Xu J., Rapior S., Jeewon R., Lumyong S., Niego A.G.T., Abeywickrama P.D., Aluthmuhandiram J.V.S., Brahamanage R.S., Brooks S., et al. The Amazing Potential of Fungi: 50 Ways We Can Exploit Fungi Industrially. Fungal Divers. 2019;97:1–136. doi: 10.1007/s13225-019-00430-9. - DOI
-
- Alastruey-Izquierdo A., Mellado E., Peláez T., Pemán J., Zapico S., Alvarez M., Rodríguez-Tudela J.L., Cuenca-Estrella M. Population-Based Survey of Filamentous Fungi and Antifungal Resistance in Spain (FILPOP Study) Antimicrob. Agents Chemother. 2013;57:3380–3387. doi: 10.1128/AAC.00383-13. - DOI - PMC - PubMed
Grants and funding
- HZI POF IV Cooperativity and Creativity Project Call/Helmholtz Centre for Infection Research
- Project-ID 490821847/Deutsche Forschungsgemeinschaft
- MSCA-RISE grant no. 101008129, Acronym: MYCOBIOMICS/European Union's H2020 Research and Innovation Staff Exchange program
- SEA-Europe Grant number JFS20ST-127, Acronym: Antiviralfun/Southeast Asia - Europe Joint Funding Scheme
LinkOut - more resources
Full Text Sources
Research Materials

