Extracellular Vesicles of the Plant Pathogen Botrytis cinerea
- PMID: 37108947
- PMCID: PMC10146736
- DOI: 10.3390/jof9040495
Extracellular Vesicles of the Plant Pathogen Botrytis cinerea
Abstract
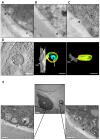
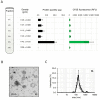
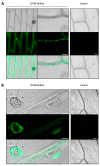
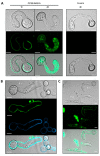
Fungal secretomes are known to contain a multitude of components involved in nutrition, cell growth or biotic interactions. Recently, extra-cellular vesicles have been identified in a few fungal species. Here, we used a multidisciplinary approach to identify and characterize extracellular vesicles produced by the plant necrotroph Botrytis cinerea. Transmission electron microscopy of infectious hyphae and hyphae grown in vitro revealed extracellular vesicles of various sizes and densities. Electron tomography showed the co-existence of ovoid and tubular vesicles and pointed to their release via the fusion of multi-vesicular bodies with the cell plasma membrane. The isolation of these vesicles and exploration of their protein content using mass spectrometry led to the identification of soluble and membrane proteins involved in transport, metabolism, cell wall synthesis and remodeling, proteostasis, oxidoreduction and traffic. Confocal microscopy highlighted the capacity of fluorescently labeled vesicles to target cells of B. cinerea, cells of the fungus Fusarium graminearum, and onion epidermal cells but not yeast cells. In addition, a specific positive effect of these vesicles on the growth of B. cinerea was quantified. Altogether, this study broadens our view on the secretion capacity of B. cinerea and its cell-to-cell communication.
Keywords: EV; cell–cell communication; electron/confocal microscopy; fungus; proteomics; secretion; tomography.
Conflict of interest statement
B.M. was employed by the company Bayer SAS. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- De Vallée A., Bally P., Bruel C., Chandat L., Choquer M., Dieryckx C., Dupuy J.-W., Kaiser S., Latorse M.-P., Loisel E., et al. A Similar Secretome Disturbance as a Hallmark of Non-pathogenic Botrytis cinerea ATMT-Mutants? Front. Microbiol. 2019;10:2829. doi: 10.3389/fmicb.2019.02829. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

