First Description of Non-Enzymatic Radical-Generating Mechanisms Adopted by Fomitiporia mediterranea: An Unexplored Pathway of the White Rot Agent of the Esca Complex of Diseases
- PMID: 37108951
- PMCID: PMC10143301
- DOI: 10.3390/jof9040498
First Description of Non-Enzymatic Radical-Generating Mechanisms Adopted by Fomitiporia mediterranea: An Unexplored Pathway of the White Rot Agent of the Esca Complex of Diseases
Abstract
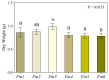
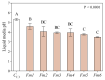
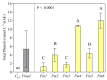
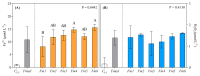
Fomitiporia mediterranea (Fmed) is the primary Basidiomycota species causing white rot in European vineyards affected by the Esca complex of diseases (ECD). In the last few years, an increasing number of studies have highlighted the importance of reconsidering the role of Fmed in ECD etiology, justifying an increase in research interest related to Fmed's biomolecular pathogenetic mechanisms. In the context of the current re-evaluation of the binary distinction (brown vs. white rot) between biomolecular decay pathways induced by Basidiomycota species, our research aims to investigate the potential for non-enzymatic mechanisms adopted by Fmed, which is typically described as a white rot fungus. Our results demonstrate how, in liquid culture reproducing nutrient restriction conditions often found in wood, Fmed can produce low molecular weight compounds, the hallmark of the non-enzymatic "chelator-mediated Fenton" (CMF) reaction, originally described for brown rot fungi. CMF reactions can redox cycle with ferric iron, generating hydrogen peroxide and ferrous iron, necessary reactants leading to hydroxyl radical (•OH) production. These observations led to the conclusion that a non-enzymatic radical-generating CMF-like mechanism may be utilized by Fmed, potentially together with an enzymatic pool, to contribute to degrading wood constituents; moreover, indicating significant variability between strains.
Keywords: CMF; Fmed; GTDs; ferric iron; grapevine; phenolates; •OH.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Wood Degradation by Fomitiporia mediterranea M. Fischer: Exploring Fungal Adaptation Using Metabolomic Networking.J Fungi (Basel). 2023 Apr 30;9(5):536. doi: 10.3390/jof9050536. J Fungi (Basel). 2023. PMID: 37233247 Free PMC article.
-
Wood degradation by Fomitiporia mediterranea M. Fischer: Physiologic, metabolomic and proteomic approaches.Front Plant Sci. 2022 Sep 26;13:988709. doi: 10.3389/fpls.2022.988709. eCollection 2022. Front Plant Sci. 2022. PMID: 36226293 Free PMC article.
-
Bacteria associated with wood tissues of Esca-diseased grapevines: functional diversity and synergy with Fomitiporia mediterranea to degrade wood components.Environ Microbiol. 2021 Oct;23(10):6104-6121. doi: 10.1111/1462-2920.15676. Epub 2021 Jul 31. Environ Microbiol. 2021. PMID: 34288352 Free PMC article.
-
White Rot Fungi (Hymenochaetales) and Esca of Grapevine: Insights from Recent Microbiome Studies.J Fungi (Basel). 2021 Sep 17;7(9):770. doi: 10.3390/jof7090770. J Fungi (Basel). 2021. PMID: 34575808 Free PMC article. Review.
-
Pollutant degradation by white rot fungi.Rev Environ Contam Toxicol. 1994;138:49-72. doi: 10.1007/978-1-4612-2672-7_3. Rev Environ Contam Toxicol. 1994. PMID: 7938784 Review.
Cited by
-
Whole-Genome Characterization of Inonotus hispidus from Ulmus macrocarpa and Its Comparative Genomics with Strains from Morus alba and Acer truncatum.J Fungi (Basel). 2025 Apr 29;11(5):346. doi: 10.3390/jof11050346. J Fungi (Basel). 2025. PMID: 40422680 Free PMC article.
-
Genome analysis of the esca-associated Basidiomycetes Fomitiporia mediterranea, Fomitiporia polymorpha, Inonotus vitis, and Tropicoporus texanus reveals virulence factor repertoires characteristic of white-rot fungi.G3 (Bethesda). 2024 Oct 7;14(10):jkae189. doi: 10.1093/g3journal/jkae189. G3 (Bethesda). 2024. PMID: 39141591 Free PMC article.
-
Biochemical characterization of wood decay and metabolization of phenolic compounds by causal fungi of grapevine trunk diseases.PLoS One. 2025 Apr 16;20(4):e0315412. doi: 10.1371/journal.pone.0315412. eCollection 2025. PLoS One. 2025. PMID: 40238810 Free PMC article.
References
-
- Mondello V., Songy A., Battiston E., Pinto C., Coppin C., Trotel-Aziz P., Clément C., Mugnai L., Fontaine F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018;102:1189–1217. doi: 10.1094/PDIS-08-17-1181-FE. - DOI - PubMed
-
- Guérin-Dubrana L., Fontaine F., Mugnai L. Grapevine Trunk Disease in European and Mediterranean Vineyards: Occurrence, Distribution and Associated Disease-Affecting Cultural Factors. Phytopathol. Mediterr. 2019;58:49–71. doi: 10.14601/Phytopathol_Mediterr-25153. - DOI
-
- Kenfaoui J., Radouane N., Mennani M., Tahiri A., El Ghadraoui L., Belabess Z., Fontaine F., El Hamss H., Amiri S., Lahlali R., et al. A Panoramic View on Grapevine Trunk Diseases Threats: Case of Eutypa Dieback, Botryosphaeria Dieback, and Esca Disease. J. Fungi. 2022;8:595. doi: 10.3390/jof8060595. - DOI - PMC - PubMed
-
- Calzarano F., Osti F., D’Agostino V., Pepe A., Di Marco S. Mixture of Calcium, Magnesium and Seaweed Affects Leaf Phytoalexin Contents and Grape Ripening on Vines with Grapevine Leaf Stripe Disease. Phytopathol. Mediterr. 2017;56:445–457. doi: 10.14601/Phytopathol_Mediterr-22023. - DOI
-
- Lorrain B., Ky I., Pasquier G., Jourdes M., Dubrana L.G., Gény L., Rey P., Donèche B., Teissedre P.L. Effect of Esca Disease on the Phenolic and Sensory Attributes of Cabernet Sauvignon Grapes, Musts and Wines. Aust. J. Grape Wine Res. 2012;18:64–72. doi: 10.1111/j.1755-0238.2011.00172.x. - DOI
LinkOut - more resources
Full Text Sources

