Baseline CTC Count as a Predictor of Long-Term Outcomes in High-Risk Prostate Cancer
- PMID: 37108995
- PMCID: PMC10144132
- DOI: 10.3390/jpm13040608
Baseline CTC Count as a Predictor of Long-Term Outcomes in High-Risk Prostate Cancer
Abstract
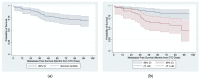
The aim of the present study was to verify whether the baseline circulating tumor cell (CTC) count might serve as a predictor of overall survival (OS) and metastasis-free survival (MFS) in patients with high-risk prostate cancer (PCa) during a follow-up period of at least 5 years. CTCs were enumerated using three different assay formats in 104 patients: the CellSearch® system, EPISPOT assay and GILUPI CellCollector. A total of 57 (55%) patients survived until the end of the follow-up period, with a 5 year OS of 66% (95% CI: 56-74%). The analysis of univariate Cox proportional hazard models identified a baseline CTC count ≥ 1, which was determined with the CellSearch® system, a Gleason sum ≥ 8, cT ≥ 2c and metastases at initial diagnosis as significant predictors of a worse OS in the entire cohort. The CTC count ≥ 1 was also the only significant predictor of a worse OS in a subset of 85 patients who presented with localized PCa at the baseline. The baseline CTC number did not affect the MFS. In conclusion, the baseline CTC count can be considered a determinant of survival in high-risk PCa and also in patients with a localized disease. However, determining the prognostic value of the CTC count in patients with localized PCa would optimally require longitudinal monitoring of this parameter.
Keywords: circulating tumor cells; metastasis-free survival; overall survival; prognosis; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Perez-Gracia J.L., Sanmamed M.F., Bosch A., Patiño-Garcia A., Schalper K.A., Segura V., Bellmunt J., Tabernero J., Sweeney C.J., Choueiri T.K., et al. Strategies to design clinical studies to identify predictive biomarkers in cancer research. Cancer Treat. Rev. 2017;53:79–97. doi: 10.1016/j.ctrv.2016.12.005. - DOI - PubMed
-
- Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T., Mason M., Matveev V., Wiegel T., Zattoni F., et al. EAU guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. - DOI - PubMed
LinkOut - more resources
Full Text Sources

