Revascularization and Medical Therapy for Chronic Coronary Syndromes: Lessons Learnt from Recent Trials, a Literature Review
- PMID: 37109169
- PMCID: PMC10141707
- DOI: 10.3390/jcm12082833
Revascularization and Medical Therapy for Chronic Coronary Syndromes: Lessons Learnt from Recent Trials, a Literature Review
Abstract
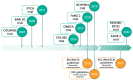
Stable coronary artery disease (CAD) has recently been replaced by a new entity described as chronic coronary syndrome (CCS). This new entity has been developed based on a better understanding of the pathogenesis, the clinical characteristics, and the morbi-mortality associated to this condition as part of the dynamic spectrum of CAD. This has significant implications in the clinical management of CCS patients, that ranges from lifestyle adaptation, medical therapy targeting all the elements contributing to CAD progression (i.e., platelet aggregation, coagulation, dyslipidaemia, and systemic inflammation), to invasive strategies (i.e., revascularization). CCS is the most frequent presentation of coronary artery disease which is the first cardiovascular disease worldwide. Medical therapy is the first line therapy for these patients; however, revascularization and especially percutaneous coronary intervention remains beneficial for some of them. European and American guidelines on myocardial revascularization were released in 2018 and 2021, respectively. These guidelines provide different scenarios to help physicians choose the optimal therapy for CCS patients. Recently, several trials focusing on CCS patients have been published. We sought to synthetize the place of revascularization in CCS patients according to the latest guidelines, the lessons learnt from recent trials on revascularization and medical therapy, and future perspectives.
Keywords: CCS; chronic coronary syndromes; coronary artery disease; revascularization.
Conflict of interest statement
F.P. reports research, consulting, and speaking fees from Astra-Zeneca, Bayer, BBraun, Biotronik, BMS-Pfizer Alliance, Boston Scientific, Sanofi, and Servier outside the submitted work. The other authors declare that they have no known conflict of interest.
Figures

References
-
- Knuuti J. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) Russ. J. Cardiol. 2020;25:119–180. doi: 10.15829/1560-4071-2020-2-3757. - DOI
-
- Lawton J.S., Tamis-Holland J.E., Bangalore S., Bates E.R., Beckie T.M., Bischoff J.M., Bittl J.A., Cohen M.G., DiMaio J.M., Don C.W., et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines n.d. Circulation. 2022;79:e21–e129. doi: 10.1161/CIR.0000000000001038. - DOI - PubMed
-
- Al-Lamee R., Thompson D., Dehbi H.-M., Sen S., Tang K., Davies J., Keeble T., Mielewczik M., Kaprielian R., Malik I.S., et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet. 2018;391:31–40. doi: 10.1016/S0140-6736(17)32714-9. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

