Effective Combination of the Metal Centers in MOF-Based Materials toward Sustainable Oxidation Catalysts
- PMID: 37109968
- PMCID: PMC10145539
- DOI: 10.3390/ma16083133
Effective Combination of the Metal Centers in MOF-Based Materials toward Sustainable Oxidation Catalysts
Abstract
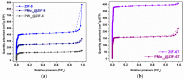
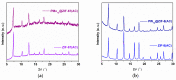
A successful encapsulation of Keggin-type polyoxomolybdate (H3[PMo12O40], PMo12) into metal-organic framework (MOF) materials with an identical framework but distinct metal centers (ZIF-8 with Zn2+ and ZIF-67 with Co2+) was accomplished by a straightforward room-temperature procedure. The presence of Zn2+ in the composite material PMo12@ZIF-8 instead of Co2+ in PMo12@ZIF-67 caused a remarkable increase in the catalytic activity that achieved a total oxidative desulfurization of a multicomponent model diesel under moderate and friendly conditions (oxidant: H2O2 and solvent: ionic liquid, IL). Interestingly, the parent ZIF-8-based composite with the Keggin-type polyoxotungstate (H3[PW12O40], PW12), PW12@ZIF-8, did not show the relevant catalytic activity. The ZIF-type supports present an appropriate framework to accommodate active polyoxometalates (POMs) into their cavities without leaching, but the nature of the metallic center from the POM and the metal present in the ZIF framework were vital for the catalytic performance of the composite materials.
Keywords: heterogeneous catalysis; nanocomposite material; oxidative desulfurization; polyoxometalates; zeolitic imidazolate frameworks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Lindqvist@Nanoporous MOF-Based Catalyst for Effective Desulfurization of Fuels.Nanomaterials (Basel). 2022 Aug 22;12(16):2887. doi: 10.3390/nano12162887. Nanomaterials (Basel). 2022. PMID: 36014754 Free PMC article.
-
Ionic Liquid-Based Polyoxometalate Incorporated at ZIF-8: A Sustainable Catalyst to Combine Desulfurization and Denitrogenation Processes.Molecules. 2022 Mar 5;27(5):1711. doi: 10.3390/molecules27051711. Molecules. 2022. PMID: 35268812 Free PMC article.
-
Improving the Catalytic Performance of Keggin [PW12O40]3- for Oxidative Desulfurization: Ionic Liquids versus SBA-15 Composite.Materials (Basel). 2018 Jul 12;11(7):1196. doi: 10.3390/ma11071196. Materials (Basel). 2018. PMID: 30002316 Free PMC article.
-
Rapid Fabrication of Biocomposites by Encapsulating Enzymes into Zn-MOF-74 via a Mild Water-Based Approach.ACS Appl Mater Interfaces. 2021 Nov 10;13(44):52014-52022. doi: 10.1021/acsami.1c09052. Epub 2021 Jul 7. ACS Appl Mater Interfaces. 2021. PMID: 34232015 Review.
-
Critical Review, Recent Updates on Zeolitic Imidazolate Framework-67 (ZIF-67) and Its Derivatives for Electrochemical Water Splitting.Adv Mater. 2022 Mar;34(11):e2107072. doi: 10.1002/adma.202107072. Epub 2022 Feb 2. Adv Mater. 2022. PMID: 34846082 Review.
Cited by
-
Evaluation of Phosphopolyoxometalates with Mixed Addenda (Mo, W, V) as Corrosion Inhibitors for Steels.Materials (Basel). 2023 Dec 11;16(24):7600. doi: 10.3390/ma16247600. Materials (Basel). 2023. PMID: 38138742 Free PMC article.
-
Silicotungstate@ZIF-67 as an effective catalyst for an extraction and oxidative desulfurization system.RSC Adv. 2024 Nov 18;14(49):36622-36632. doi: 10.1039/d4ra06736c. eCollection 2024 Nov 11. RSC Adv. 2024. PMID: 39559581 Free PMC article.
References
-
- Abas N., Kalair A., Khan N. Review of fossil fuels and future energy technologies. Futures. 2015;69:31–49. doi: 10.1016/j.futures.2015.03.003. - DOI
-
- Ismagilov Z., Yashnik S., Kerzhentsev M., Parmon V., Bourane A., Al-Shahrani F.M., Hajji A.A., Koseoglu O.R. Oxidative Desulfurization of Hydrocarbon Fuels. Catal. Rev. 2011;53:199–255. doi: 10.1080/01614940.2011.596426. - DOI
-
- Samokhvalov A. Desulfurization of Real and Model Liquid Fuels Using Light: Photocatalysis and Photochemistry. Catal. Rev. 2012;54:281–343. doi: 10.1080/01614940.2012.650958. - DOI
-
- Stanislaus A., Marafi A., Rana M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today. 2010;153:1–68. doi: 10.1016/j.cattod.2010.05.011. - DOI
-
- Chandra Srivastava V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012;2:759–783. doi: 10.1039/C1RA00309G. - DOI
LinkOut - more resources
Full Text Sources

