Surfactant-Free Decellularization of Porcine Auricular Cartilage Using Liquefied Dimethyl Ether and DNase
- PMID: 37110010
- PMCID: PMC10146022
- DOI: 10.3390/ma16083172
Surfactant-Free Decellularization of Porcine Auricular Cartilage Using Liquefied Dimethyl Ether and DNase
Abstract
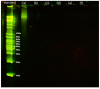
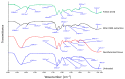
The most common decellularization method involves lipid removal using surfactant sodium dodecyl sulfate (SDS) and DNA fragmentation using DNase, and is associated with residual SDS. We previously proposed a decellularization method for the porcine aorta and ostrich carotid artery using liquefied dimethyl ether (DME), which is free from the concerns associated with SDS residues, instead of SDS. In this study, the DME + DNase method was tested on crushed porcine auricular cartilage tissues. Unlike with the porcine aorta and the ostrich carotid artery, it is important to degas the porcine auricular cartilage using an aspirator before DNA fragmentation. Although approximately 90% of the lipids were removed using this method, approximately 2/3 of the water was removed, resulting in a temporary Schiff base reaction. The amount of residual DNA in the tissue was approximately 27 ng/mg dry weight, which is lower than the regulatory value of 50 ng/mg dry weight. Hematoxylin and eosin staining confirmed that cell nuclei were removed from the tissue. Residual DNA fragment length assessment by electrophoresis confirmed that the residual DNA was fragmented to less than 100 bp, which was lower than the regulatory limit of 200 bp. By contrast, in the uncrushed sample, only the surface was decellularized. Thus, although limited to a sample size of approximately 1 mm, liquefied DME can be used to decellularize porcine auricular cartilage. Thus, liquefied DME, with its low persistence and high lipid removal capacity, is an effective alternative to SDS.
Keywords: decellularization; extraction; liquefied gas; scaffold; subcritical fluid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; or decision to publish the results.
Figures








Similar articles
-
Extraction and Separation of Natural Products from Microalgae and Other Natural Sources Using Liquefied Dimethyl Ether, a Green Solvent: A Review.Foods. 2024 Jan 22;13(2):352. doi: 10.3390/foods13020352. Foods. 2024. PMID: 38275719 Free PMC article. Review.
-
Surfactant-Free Decellularization of Porcine Aortic Tissue by Subcritical Dimethyl Ether.ACS Omega. 2021 May 14;6(20):13417-13425. doi: 10.1021/acsomega.1c01549. eCollection 2021 May 25. ACS Omega. 2021. PMID: 34056489 Free PMC article.
-
Tensile Strength of Porcine Aorta Decellularized with Liquefied Dimethyl Ether and DNase.ACS Omega. 2022 Sep 13;7(38):34449-34453. doi: 10.1021/acsomega.2c04103. eCollection 2022 Sep 27. ACS Omega. 2022. PMID: 36188255 Free PMC article.
-
Decellularization of kidney tissue: comparison of sodium lauryl ether sulfate and sodium dodecyl sulfate for allotransplantation in rat.Cell Tissue Res. 2021 Nov;386(2):365-378. doi: 10.1007/s00441-021-03517-5. Epub 2021 Aug 23. Cell Tissue Res. 2021. PMID: 34424397
-
Decellularization of porcine carotid arteries using low-concentration sodium dodecyl sulfate.Int J Artif Organs. 2021 Jul;44(7):497-508. doi: 10.1177/0391398820975420. Epub 2020 Nov 23. Int J Artif Organs. 2021. PMID: 33222583
Cited by
-
Extraction and Separation of Natural Products from Microalgae and Other Natural Sources Using Liquefied Dimethyl Ether, a Green Solvent: A Review.Foods. 2024 Jan 22;13(2):352. doi: 10.3390/foods13020352. Foods. 2024. PMID: 38275719 Free PMC article. Review.
-
Recent advances in bionic scaffolds for cartilage tissue engineering.Front Bioeng Biotechnol. 2025 Jul 11;13:1625550. doi: 10.3389/fbioe.2025.1625550. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40718699 Free PMC article. Review.
-
Determination of residual DNA in decellularised aortas- towards fluorescence-based quantification of DNA purified by various methods.Mol Biol Rep. 2025 Jul 8;52(1):682. doi: 10.1007/s11033-025-10755-1. Mol Biol Rep. 2025. PMID: 40627208 Free PMC article.
References
-
- Seror J., Merkher Y., Kampf N., Collinson L., Day A.J., Maroudas A., Klein J. Articular cartilage proteoglycans as boundary lubricants: Structure and frictional interaction of surface-attached hyaluronan and hyaluronan–aggrecan complexes. Biomacromolecules. 2011;12:3432–3443. doi: 10.1021/bm2004912. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

