Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks
- PMID: 37110318
- PMCID: PMC10146995
- DOI: 10.3390/microorganisms11040895
Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks
Abstract
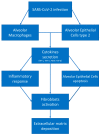
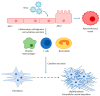
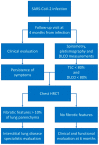
Idiopathic pulmonary fibrosis (IPF) is considered the paradigmatic example of chronic progressive fibrosing disease; IPF does not result from a primary immunopathogenic mechanism, but immune cells play a complex role in orchestrating the fibrosing response. These cells are activated by pathogen-associated or danger-associated molecular patterns generating pro-fibrotic pathways or downregulating anti-fibrotic agents. Post-COVID pulmonary fibrosis (PCPF) is an emerging clinical entity, following SARS-CoV-2 infection; it shares many clinical, pathological, and immune features with IPF. Similarities between IPF and PCPF can be found in intra- and extracellular physiopathological pro-fibrotic processes, genetic signatures, as well as in the response to antifibrotic treatments. Moreover, SARS-CoV-2 infection can be a cause of acute exacerbation of IPF (AE-IPF), which can negatively impact on IPF patients' prognosis. In this narrative review, we explore the pathophysiological aspects of IPF, with particular attention given to the intracellular signaling involved in the generation of fibrosis in IPF and during the SARS-CoV-2 infection, and the similarities between IPF and PCPF. Finally, we focus on COVID-19 and IPF in clinical practice.
Keywords: COVID-19; SARS-CoV-2; idiopathic pulmonary fibrosis; post-COVID-19 pulmonary fibrosis; pulmonary fibrosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Raghu G., Remy-Jardin M., Richeldi L., Thomson C.C., Inoue Y., Johkoh T., Kreuter M., Lynch D.A., Maher T.M., Martinez F.J., et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

